Abstract
Isolated gastric IgG4-related disease (IgG4-RD) is a very rare tumefactive inflammatory condition, with only a few cases reported to date. A 48-year-old woman was incidentally found to have a subepithelial tumor in the stomach. Given a presumptive diagnosis of gastrointestinal stromal tumor or neuroendocrine tumor, she underwent wedge resection. The lesion was vaguely nodular and mainly involved the submucosa and proper muscle layer. Microscopically, all classical features of type I autoimmune pancreatitis including lymphoplasmacytic infiltration, storiform fibrosis, obliterative phlebitis, and numerous IgG4-positive plasma cells were seen. She had no evidence of IgG4-RD in other organs. Although very rare, IgG4-RD should be considered one of the differential diagnoses in the setting of gastric wall thickening or subepithelial mass-like lesion. Deep biopsy with awareness of this entity might avoid unnecessary surgical intervention.
IgG4-related disease (IgG4-RD) is a recently recognized inflammatory condition characterized by several clinico-pathologic features: a tendency to form mass-like lesions, dense lymphoplasmacytic infiltration, storiform fibrosis, obliterative phlebitis, increased IgG4-expressing plasma cells, and often but not always elevated serum IgG4 level [1-4]. IgG4-RD can affect multiple organs simultaneously or can present as a solitary, mass-like lesion. When IgG4-RD presents as a solitary, mass-like lesion, it might be misinterpreted clinically or radiologically as a neoplasm, resulting in overtreatment. IgG4-RD usually affects the pancreas, biliary tree, liver, salivary glands, lacrimal glands, and retroperitoneum; however, involvement of the gastrointestinal (GI) tract is very rare, and diagnostic criteria have not yet been well established. A recent review proposed two types of IgG4-RD of the GI tract. One is diffuse wall thickening, and the other is polyp or mass-like lesion [5]. To date, there have been eight IgG4-RDs reported in the stomach regardless of the presence in other organs. Here, we describe the ninth case of IgG4-RD in the stomach, which presented as an isolated mass-like lesion without involvement of any other organ.
A 48-year-old, previously healthy woman was found to have a subepithelial tumor during health screening endoscopy (Fig. 1A). Abdominal computed tomography demonstrated a 3.6× 2.2 cm, well-defined, solid, enhancing submucosal mass on the posterior wall of the stomach midbody (Fig. 1B). Radiologic differential diagnoses included GI stromal tumor and neuroendocrine tumor. No remarkable findings were observed in other organs. Seven years ago, she had undergone modified radical mastectomy for breast cancer. There was no further history, symptoms, or signs of systemic disease, and laboratory tests were unremarkable. Serum IgG4 level was not measured preoperatively. Given a presumptive diagnosis of submucosal neoplasm, wedge resection was performed.
Grossly, the lesion was a poorly circumscribed, yellowish grey, fusiform mass involving the area from the submucosa to subserosa (Fig. 2A). The overlying mucosa was intact, and there was no ulceration. Microscopically, the mass showed marked fibrosis, often in a storiform pattern of many lymphoid follicles with well-formed germinal centers, and diffuse inflammatory cell infiltration. The infiltrated inflammatory cells were mainly lymphocytes and plasma cells, but some eosinophils were also found (Fig. 2B–D). Obliterative phlebitis was occasionally observed in elastic staining (Fig. 2E). There were numerous IgG4-positive cells throughout the lesion, and the number of IgG4-positive plasma cells was up to 210 per high-power field (Fig. 2F). The IgG4 to IgG-positive cell number ratio was about 85%; however, there were only a few IgG4-positive cells in the mucosa. There was no significant myofibroblastic proliferation or immunostaining for anaplastic lymphoma kinase; therefore, the possibility of inflammatory myofibroblastic tumor was excluded. We concluded that this lesion was IgG4-RD. The patient’s postoperative course was uneventful, and she was discharged without any complications. No recurrence was observed during the 10-month follow-up period.
Here, we described a case of isolated gastric IgG4-RD presenting as a fusiform mural mass mimicking neoplasm, such as GI stromal tumor or neuroendocrine tumor. To the best of our knowledge, this is the ninth case of gastric IgG4-RD. Histologically, this case demonstrated all the important features of IgG4-RD, including dense lymphoplasmacytic infiltration, storiform fibrosis, obliterative phlebitis, and abundant IgG4-positive cells. Although other diagnostic criteria, such as elevated serum IgG4 level or response to steroid therapy, could not be confirmed due to the clinical presentation, typical histopathologic features led us to consider IgG4-RD.
Increased IgG4-positive plasma cells can be seen in other organs and in many conditions, including non-specific chronic inflammation, lymphoma, and other malignancies [4,6-8]. However, these lesions lack other characteristic histopathologic findings, such as storiform fibrosis and oblierative phlebitis, as has been described in the consensus statement on the pathology of IgG4-RD [4]. Although abundant IgG4-positive plasma cell infiltration is not uncommon in the GI tract in the setting of autoimmune pancreatitis, the simple presence of IgG4-positive cells does not justify a diagnosis of IgG4-RD in the absence of other gross and microscopic features, such as tumefactive nature, storiform fibrosis, and obliterative phlebitis.
Including the present case, there have been nine cases of massforming IgG4-RD in the stomach [9-14]. A case of probable IgG4-RD that presented as a gastric ulcer has also been reported [15,16], but we excluded this case from the present review. As is summarized in Table 1, most gastric IgG4-RD was detected in middle age (mean, 58.8 years; range, 45 to 75 years), and men and women were affected equally, although the total number of patients is likely too small to reveal any meaningful data. Seven patients had solitary nodules or masses, whereas two patients had multiple polyps or nodules. The two patients with multiple lesions also had autoimmune pancreatitis and autoimmune polyendocrinopathy, respectively. Four of the seven cases showing a solitary lesion had no sign of multi-organ involvement. Most cases of gastric IgG4-RD (six of nine) involved the submucosal layer of the gastric body. Proper muscle or mucosa was variably involved. Serum IgG4 was increased only in patients with associated autoimmune pancreatitis. Most gastric IgG4-RD patients were treated surgically except for one patient with autoimmune pancreatitis who was treated with steroid.
Steroid treatment is the first therapeutic option in IgG4-RD [17], but all reported isolated gastric IgG4-RD cases were surgically resected, presumably because they are rare and difficult to diagnose without pathologic examination of a resected specimen. Unnecessary surgery might be avoided if the possibility of IgG4-RD is kept in mind and careful pathologic evaluation including IgG4 immunostaining is performed on a deep biopsy obtained using endoscopic ultrasonography.
REFERENCES
1. Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001; 344:732–8.

2. Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003; 38:982–4.

4. Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012; 25:1181–92.
5. Koizumi S, Kamisawa T, Kuruma S, et al. Immunoglobulin G4-related gastrointestinal diseases, are they immunoglobulin G4-related diseases? World J Gastroenterol. 2013; 19:5769–74.

6. Strehl JD, Hartmann A, Agaimy A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol. 2011; 64:237–43.

7. Zhang L, Notohara K, Levy MJ, Chari ST, Smyrk TC. IgG4-positive plasma cell infiltration in the diagnosis of autoimmune pancreatitis. Mod Pathol. 2007; 20:23–8.

8. Witkiewicz AK, Kennedy EP, Kennyon L, Yeo CJ, Hruban RH. Synchronous autoimmune pancreatitis and infiltrating pancreatic ductal adenocarcinoma: case report and review of the literature. Hum Pathol. 2008; 39:1548–51.

9. Na KY, Sung JY, Jang JY, et al. Gastric nodular lesion caused by IgG4-related disease. Pathol Int. 2012; 62:716–8.

10. Baez JC, Hamilton MJ, Bellizzi A, Mortelé KJ. Gastric involvement in autoimmune pancreatitis: MDCT and histopathologic features. JOP. 2010; 11:610–3.
11. Kaji R, Okabe Y, Ishida Y, et al. Autoimmune pancreatitis presenting with IgG4-positive multiple gastric polyps. Gastrointest Endosc. 2010; 71:420–2.

12. Chetty R, Serra S, Gauchotte G, Märkl B, Agaimy A. Sclerosing nodular lesions of the gastrointestinal tract containing large numbers of IgG4 plasma cells. Pathology. 2011; 43:31–5.

13. Rollins KE, Mehta SP, O’Donovan M, Safranek PM. Gastric IgG4-related autoimmune fibrosclerosing pseudotumour: a novel location. ISRN Gastroenterol. 2011; 2011:873087.

14. Kim DH, Kim J, Park DH, et al. Immunoglobulin G4-related inflammatory pseudotumor of the stomach. Gastrointest Endosc. 2012; 76:451–2.

15. Bateman AC, Sommerlad M, Underwood TJ. Chronic gastric ulceration: a novel manifestation of IgG4-related disease? J Clin Pathol. 2012; 65:569–70.

Fig. 1.
Endoscopic and abdominal computed tomography scan images. (A) Localized smooth elevation of the gastric mucosa without mucosal fold abnormality. (B) A well-defined, solid, enhancing mass measuring 3.6 × 2.2 cm at the posterior wall of the stomach midbody (arrow).
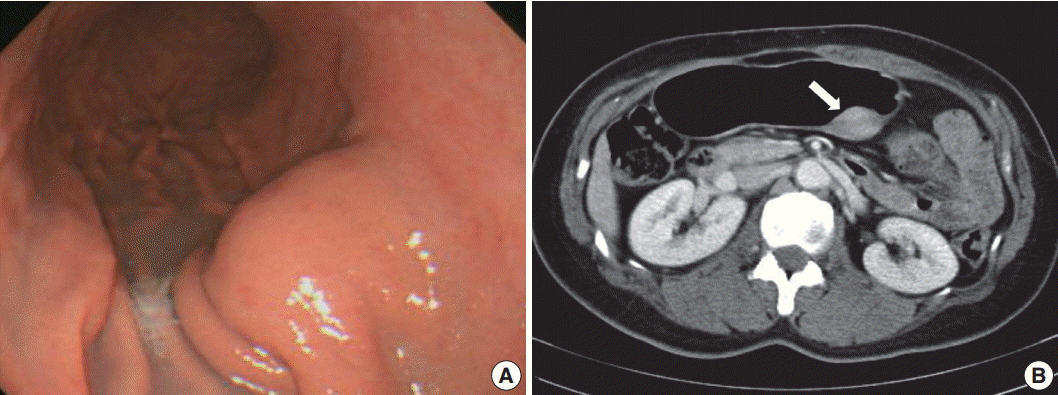
Fig. 2.
Gross and microscopic appearance of the resected specimen. (A) An ill-defined, yellowish grey mass involves the full thickness of the gastric wall except the mucosa. (B) The mass is not encapsulated and is filled with fibrotic tissue and multiple lymphoid follicles. (C) Storiform fibrosis is observed between lymphoid follicles. (D) Numerous plasma cells and many eosinophils are noted in the fibrotic stroma. (E) Obliterative phlebitis is demonstrated in elastic staining (arrow). Note the residual elastic fiber of the obliterated vein (Van Gieson). (F) Numerous IgG4-positive cells are noted in the sclerotic area.
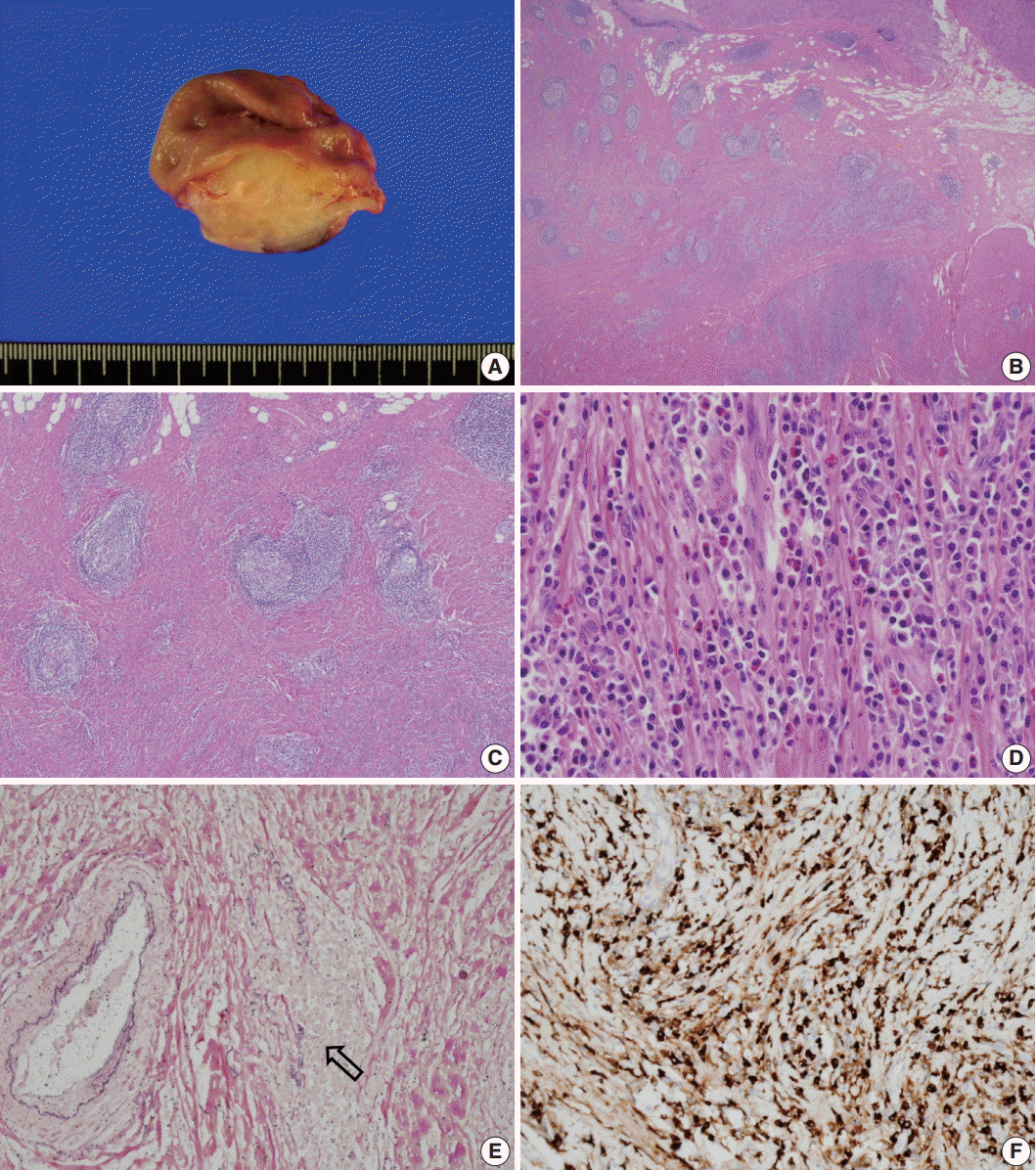
Table 1.
Clinicopathologic features of the cases of probable IgG4-related disease in the stomach
| Reference | Sex/Age (yr) | Endoscopic finding | Sites | Invovement | Serum IgG4 | Procedure | Associated condition |
|---|---|---|---|---|---|---|---|
| Baez et al. [10] | M/58 | Nodule, 1.4 cm | Fundus and body | Mucosa | Normal | Steroid | AIP, IgG4-related sialadenitis |
| Kaji et al. [11] | M/74 | Mutiple polyps with erosion and redness | Body | Mucosa | Increased | NA | AIP |
| Chetty et al. [12] | F/45 | Nodule, 1.5 cm | Fundus | Submucosa | Normal | WR | Raynaud’s disease |
| Chetty et al. [12] | M/60 | Multiple nodules, up to 2.2 cm | Antrum | Proper muscle to submucosa | NA | DG | Autoimmune polyendocrinopathy |
| Rollins et al. [13] | F/75 | Polypoid lesion, 5.6 cm | Body | Submucosa | NA | WR | None |
| Na et al. [9] | M/56 | Nodule, 0.8 cm | Low body | Submucosa | NA | ESD | Type 2 diabetes mellitus |
| Kim et al. [14] | F/59 | Mass, 3.3 cm | Midbody | Proper muscle | Normal | WR | None |
| Kim et al. [14] | F/54 | Mass, 2.1 cm | NA | Proper muscle to submucosa | Normal | WR | None |
| Present case | F/48 | Mass, 3.6 cm | Midbody | Submucosa to subserosa | NA | WR | None |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download