Abstract
Nonvariceal upper gastrointestinal (GI) bleeding is one of the most common reasons for hospitalization and a major cause of morbidity and mortality worldwide. Recently developed endoscopic devices and supporting apparatuses can achieve endoscopic hemostasis with greater safety and efficiency. With these advancements in technology and technique, gastroenterologists should have no concerns regarding the management of acute upper GI bleeding, provided that they are well prepared and trained. However, when endoscopic hemostasis fails, endoscopy should not be continued. Rather, endoscopists should refer patients to radiologists and surgeons without any delay for evaluation regarding the appropriateness of emergency interventional radiology or surgery.
Nonvariceal upper gastrointestinal bleeding (NVUGIB) is one of the most common reasons for hospitalization and a major cause of morbidity and mortality worldwide.1 Despite improvements in the management of this condition, the mortality rate has remained unchanged, possibly due to a longer life expectancy and the corresponding higher number of comorbidities. International consensus recommendations on the management of patients with NVUGIB were published in 2010.2 In addition, an Asia-Pacific working group produced its own consensus recommendations taking into account some regional characteristics of NVUGIB. These include a high prevalence of Helicobacter pylori infection and a potential difference in drug metabolism in Asia-Pacific populations as compared to other populations worldwide.3 Recently, new endoscopic devices and apparatuses have been used for endoscopic hemostasis along with the increasingly widespread use of the endoscopic submucosal dissection (ESD) technique. In this review article, we will focus on the state of the art management of NVUGIB with a view to optimizing treatment efficacy for individual patients.
Patients presenting with upper gastrointestinal (GI) bleeding are at risk of hemodynamic shock and airway compromise. The priority is to assess the adequacy of the airway and circulation, and intravenous access should be secured with a large-bore cannula. Pulse oximetry, cardiac activity, and automated blood pressure measurements should also be monitored. It is important to identify any use of nonsteroidal anti-inflammatory drugs, anticoagulants such as warfarin, and antiplatelet medicines since these treatments can cause or exacerbate upper GI bleeding. Coagulation time and hemoglobin levels should be checked as well. All patients should also be blood typed and cross-matched for an appropriate number of units of packed red blood cells if necessary.4 Patients who have serious comorbid conditions may require hospitalization regardless of the severity of their bleeding.5 Existing well-validated scoring systems can help categorize patients with upper GI bleeding as having either a low or high risk of rebleeding and mortality. The most widely applied scoring systems are the Rockall and Blatchford scores.6,7 A primary aim of such scoring systems is to determine whether the patient requires any urgent intervention, including endoscopy.4 After endoscopic evaluation, assessment by the full Rockall scoring system is recommended because endoscopic findings, including adherent clots or visible vessels, can be combined with clinical factors to predict patients' risk of rebleeding and mortality. The Blatchford score is used to identify low-risk patients who do not require endoscopic intervention, although early endoscopy (within 24 hours of presentation) is recommended for most patients with acute upper GI bleeding. Some studies have shown that early endoscopy can decrease the length of patients' hospital stays, but no additional benefits of urgent endoscopy (within 1 to 12 hours of presentation) have been shown in terms of reducing rebleeding, surgery, or mortality.8,9,10,11 A strategy algorithm for the treatment of NVUGIB is shown in Fig. 1.
The Forrest classification is a method of stratifying upper GI hemorrhages into risk categories for mortality first published by Forrest et al.12 in 1974. It has been widely used to select patients for endoscopic treatment and predict the risk of re-bleeding. With recent advancements in hemostatic devices and agents, this classification continues to play an essential role in guiding treatment decisions (Fig. 2). Endoscopic hemostatic therapy is required for patients with hemorrhages classified as IA, IB, or IIA, although the necessity of endoscopic therapy for ulcers with adherent clots (IIB) is still debated.13
The hemoclip system is among the most popular endoscopic mechanical modalities for achieving hemostasis. Clips have also been applied to the closure of mucosal defects, fistulas, and perforation holes.14 Endoscopic clips are deployed over a bleeding site including visible vessels and commonly fall off within days to weeks. If the bleeding site is located in the gastric fundus or the posterior wall of the duodenal bulb, effective hemoclipping is relatively difficult. The deployment of hemoclips on a hard or fibrotic ulcer base is often challenging.15 Currently, hemoclip devices are rotatable and can be adjusted in the optimal direction by endoscopists. Hemoclips also come in various lengths, and shorter clips appear more effective on hard or fibrotic areas because they attach to these lesions more firmly than longer clips (Fig. 3A, B).16 Endoscopic band ligation devices, which have been used commonly for variceal bleeding, are also useful for treating NVUGIB by producing mechanical compression.17 The hood-like device of the ligation kit, which applies suction to varices, is useful for clearly visualizing the bleeding point. However, the effectiveness of this device depends on whether sufficient suction can be applied to a given lesion.
Argon plasma coagulation (APC) is a noncontact thermal method whereby ionized argon gas delivers a monopolar electrical current that effectively coagulates tissues. The most advantageous aspect of APC is its familiarity to the endoscopy unit in that endoscopists and nurses are able to employ this equipment in most situations, especially vascular ectasia and oozing from superficial lesions. In APC, a flexible catheter passed through the working channel of the scope is placed against the target area, ideally in a vertical position. The pressing of a foot pedal then triggers a synchronized flow of argon gas and electrical current from the generator, producing tissue coagulation (Fig. 3C). The depth of cauterization is thought to be limited because the coagulated tissue will gain increased resistance to the current. Various catheter types and operation modes are available with the APC device, including forced, pulsed, and precise. The variables that determine the cauterization effect can be set by endoscopists and nurses; these include the power setting (W), argon gas flow rate (L/min), distance to the tissue, and duration.18 In Korea and Japan, ESD has been widely performed for early gastric carcinoma, and the development of various hemostatic devices has accompanied its more widespread use. It has been reported that the use of hemostatic forceps together with soft coagulation is a safe and effective method of controlling upper GI ulcer bleeding (Fig. 3D, E).19 This type of device is preferred for the purpose of stopping spurting bleeding and ablating visible vessels.
Epinephrine injection is a common and effective method of achieving endoscopic hemostasis owing to its low risk, low cost, and simplicity (Fig. 3F). Epinephrine injection alone is superior to medical management alone, but is inferior to all other methods and should no longer be employed as a sole endoscopic treatment when other options are available.20 Other injectable agents such as thrombin, fibrin, cyanoacrylate glues, polidocanol, and ethanolamine are not commonly used in the treatment of NVUGIB.5 It should be kept in mind that arterial injection of adrenaline may cause severe hypertension during emergency gastroscopy.21 The most commonly used injectors have diameters of 23 or 25 G, while the needle length typically ranges from 3 to 5 mm. A special needle that is lengthadjustable between 2 and 7 mm is also available.
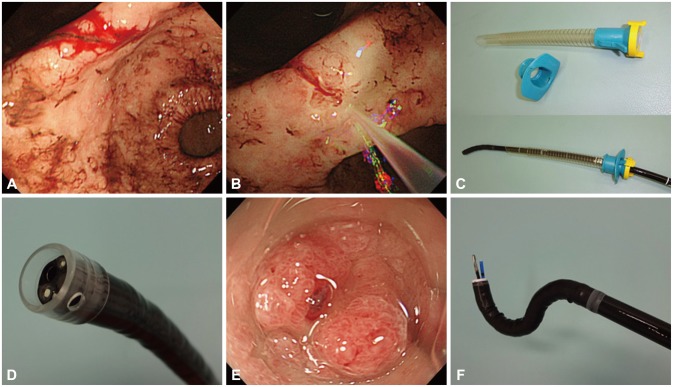
Given the difficulty in identifying the bleeding point during active bleeding, a water jet-equipped endoscope, which is widely used in ESD,22 is now indispensable for visualizing the 'culprit lesion' in NVUGIB. This scope immediately washes away the blood flow, visualizing the target point during active hemorrhage (Fig. 4A, B) and removing adherent clots to expose the visible vessel underneath. Adding a small amount of the antifoaming agent simethicone to the water bottle helps prevent any hindrance from gas bubbles. This apparatus may increase the success rate of endoscopic hemostasis in addition to reducing operation times.
A benefit of carbon dioxide (CO2) is its rapid absorption from the intestinal lumen into the blood stream. Thus, CO2 insufflation during endoscopy can significantly decrease abdominal pain and bloating both during and after the endoscopic procedure.23 CO2 insufflation is preferred for endoscopic hemostasis procedures with a longer duration. CO2 use may permit the administration of lower doses of sedative medications, which is apparently safer for patients in critical condition, and can also lead to faster recovery times from high-dependency care. However, CO2 insufflation may place patients with respiratory disorders, sleep apnea, morbid obesity, or known CO2 retention at risk of ventilator compromise. Thus, the use of conventional room air is preferred in these cases.23 CO2 insufflation is beneficial in cases of perforation due to excessive electrocautery, although efforts must be made to avoid this complication.
Aspiration pneumonia may occur in the setting of hematemesis because of massive bleeding, and this may lead to mortality. An overtube is a sleeve-like device designed to facilitate endoscopy (Fig. 4C). All overtubes have an inner diameter that is larger than the diameter of an endoscope, providing a conduit for the passage of the device into the digestive tract.24 Overtubes limit the risk of aspiration during endoscopic hemostasis. They are also beneficial for repeated intubation and withdrawal in allowing adhered mucus or clots to be wiped off the endoscope lens. In certain cases, the bleeding vessel is located in either the upper gastric body or the gastric fornix, which may not be visible owing to a large amount of blood clots. In such cases, it is necessary to switch from the conventional observation position to the right lateral decubitus position. However, many endoscopists are uncomfortable using this reversed position when performing hemostasis. Mori and colleagues25 (2013) invented an inverted overtube that can help endoscopists perform an emergency endoscopy in their conventional standing position relative to patients who are rotated to the right lateral decubitus position without changing the positions of the endoscopy unit and light source. This technique allows endoscopists to dislodge blood clots and food residue by gravity and facilitates the rapid identification of exposed vessels, resulting in a higher rate of success for hemostasis.
Endoscopic caps are commonly used in both diagnostic and therapeutic endoscopy, including mucosal resections. A variety of endoscopic caps are currently available in clinical practice, and they are effective when selected appropriately.26 It has been suggested that the application of a transparent cap to the tip of the endoscope can facilitate the approach to a tangential angle and stabilize the lesion during hemostasis (Fig. 4D, E).27 A non-randomized prospective study compared the success rates of achieving bleeding control using hemoclips with and without the aid of a transparent cap.28 In this study, hemoclipping with the cap allowed for the clipping of lesions that were situated too tangentially to be clipped without the use of the cap. Movements resulting from the patient's rapid respiration during emergency hemostasis often destabilize the positioning of the scope and hemostatic devices. In this frustrating situation, control can be achieved by pressing the cap against the lumen wall.
Performing endoscopic hemostasis is challenging in certain locations such as the lesser curvature or posterior wall of the gastric body or the cardia and the lesser curvature of the antrum. A multibending scope (GIF-2T260M; Olympus Optical, Tokyo, Japan) (Fig. 4F) that provides a second flexible section for improved positioning capability was initially developed to facilitate endoscopic mucosal resection of gastric tumors in these locations.29 Using secondary flexion, the tip of the scope can reach substantially closer to lesions in difficult to reach locations. This special scope also has two working channels, allowing for an efficient procedure whereby residual food or clots are removed by suction through one channel while a hemostatic device is inserted through the other channel.
Although endoscopic therapy usually achieves primary hemostasis, 10% to 30% of patients with NVUGIB have repeat bleeding.30,31,32 Patients in whom hemostasis is not achieved with endoscopy require transarterial embolization (TAE) or surgery. These are often elderly patients with multiple comorbidities who are poor candidates for emergency surgery.33 A large number of studies have proposed using TAE over surgery as a salvage therapy; however, there is little empirical evidence to support this recommendation.34,35 Rapid technological advances in the management of NVUGIB have been made recently via interventional radiology, and prospective randomized studies comparing the efficacy of TAE and radiological interventions after failed endoscopic hemostasis are needed. Optimal management requires a multidisciplinary team of skilled endoscopists, interventional radiologists, and GI surgeons who can integrate as a team promptly and effectively. In cases of failed endoscopic hemostasis in NVUGIB, endoscopy should not be continued and another treatment option should be decided upon without delay.
Many safe and effective devices are available for endoscopic hemostasis. With the existing advancements in technology and technique, gastroenterologists should have no concern regarding the management of acute upper GI bleeding, provided that they are well prepared and trained. However, when endoscopic hemostasis fails, endoscopy should not be continued. Rather, endoscopists should refer patients to radiologists and surgeons without delay for evaluation regarding the appropriateness of emergency interventional radiology or surgery.
References
1. Bardou M, Benhaberou-Brun D, Le Ray I, Barkun AN. Diagnosis and management of nonvariceal upper gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2012; 9:97–104. PMID: 22230903.

2. Barkun A, Bardou M, Marshall JK. Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003; 139:843–857. PMID: 14623622.

3. Sung JJ, Chan FK, Chen M, et al. Asia-Pacific Working Group consensus on non-variceal upper gastrointestinal bleeding. Gut. 2011; 60:1170–1177. PMID: 21471571.

4. Al Dhahab H, Barkun A. The acute management of nonvariceal upper gastrointestinal bleeding. Ulcers. 2012; 2012:1–8.

5. Hwang JH, Fisher DA, Ben-Menachem T, et al. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc. 2012; 75:1132–1138. PMID: 22624808.

6. Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996; 38:316–321. PMID: 8675081.

7. Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000; 356:1318–1321. PMID: 11073021.

8. Cipolletta L, Bianco MA, Rotondano G, Marmo R, Piscopo R. Outpatient management for low-risk nonvariceal upper GI bleeding: a randomized controlled trial. Gastrointest Endosc. 2002; 55:1–5. PMID: 11756905.

9. Lin HJ, Wang K, Perng CL, et al. Early or delayed endoscopy for patients with peptic ulcer bleeding. A prospective randomized study. J Clin Gastroenterol. 1996; 22:267–271. PMID: 8771420.
10. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol. 2009; 7:296.e1–302.e1. PMID: 19084483.

11. Lee JG, Turnipseed S, Romano PS, et al. Endoscopy-based triage significantly reduces hospitalization rates and costs of treating upper GI bleeding: a randomized controlled trial. Gastrointest Endosc. 1999; 50:755–761. PMID: 10570332.

12. Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974; 2:394–397. PMID: 4136718.

13. Kim KB, Yoon SM, Youn SJ. Endoscopy for nonvariceal upper gastrointestinal bleeding. Clin Endosc. 2014; 47:315–319. PMID: 25133117.

14. Technology Assessment Committee. Chuttani R, Barkun A, et al. Endoscopic clip application devices. Gastrointest Endosc. 2006; 63:746–750. PMID: 16650531.

15. Leung Ki EL, Lau JY. New endoscopic hemostasis methods. Clin Endosc. 2012; 45:224–229. PMID: 22977807.

16. Hosoe N, Imaeda H, Kashiwagi K, et al. Clinical results of endoscopic hemostasis using a short transparent hood and short hemoclips for non-variceal upper gastrointestinal bleeding. Dig Endosc. 2009; 21:93–96. PMID: 19691781.

17. Zepeda-Gómez S, Marcon NE. Endoscopic band ligation for nonvariceal bleeding: a review. Can J Gastroenterol. 2008; 22:748–752. PMID: 18818787.

18. Watson JP, Bennett MK, Griffin SM, Matthewson K. The tissue effect of argon plasma coagulation on esophageal and gastric mucosa. Gastrointest Endosc. 2000; 52:342–345. PMID: 10968847.

19. Nagata S, Kimura S, Ogoshi H, Hidaka T. Endoscopic hemostasis of gastric ulcer bleeding by hemostatic forceps coagulation. Dig Endosc. 2010; 22(Suppl 1):S22–S25. PMID: 20590766.

20. Vergara M, Calvet X, Gisbert JP. Epinephrine injection versus epinephrine injection and a second endoscopic method in high risk bleeding ulcers. Cochrane Database Syst Rev. 2007; (2):CD005584. PMID: 17443601.

21. Wu A. Arterial injection of adrenaline causing severe hypertension during emergency gastroscopy. Anaesth Intensive Care. 2013; 41:689. PMID: 23977929.
22. Tatsumi K, Uedo N, Ishihara R, et al. A water-jet videoendoscope may reduce operation time of endoscopic submucosal dissection for early gastric cancer. Dig Dis Sci. 2012; 57:2122–2129. PMID: 22451121.

23. Dellon ES, Hawk JS, Grimm IS, Shaheen NJ. The use of carbon dioxide for insufflation during GI endoscopy: a systematic review. Gastrointest Endosc. 2009; 69:843–849. PMID: 19152906.

24. ASGE Technology Committee. Tierney WM, Adler DG, et al. Overtube use in gastrointestinal endoscopy. Gastrointest Endosc. 2009; 70:828–834. PMID: 19703691.

25. Mori H, Kobara H, Fujihara S, et al. Accurate hemostasis with a new endoscopic overtube for emergency endoscopy. World J Gastroenterol. 2013; 19:2723–2726. PMID: 23674883.

27. Warneke RM, Walser E, Faruqi S, Jafri S, Bhutani MS, Raju GS. Capassisted endoclip placement for recurrent ulcer hemorrhage after repeatedly unsuccessful endoscopic treatment and angiographic embolization: case report. Gastrointest Endosc. 2004; 60:309–312. PMID: 15278071.

28. Kim JI, Kim SS, Park S, et al. Endoscopic hemoclipping using a transparent cap in technically difficult cases. Endoscopy. 2003; 35:659–662. PMID: 12929060.

29. Isshi K, Tajiri H, Fujisaki J, et al. The effectiveness of a new multibending scope for endoscopic mucosal resection. Endoscopy. 2004; 36:294–297. PMID: 15057677.

30. Bjorkman DJ, Zaman A, Fennerty MB, Lieberman D, Disario JA, Guest-Warnick G. Urgent vs. elective endoscopy for acute non-variceal upper-GI bleeding: an effectiveness study. Gastrointest Endosc. 2004; 60:1–8. PMID: 15229417.

31. Church NI, Palmer KR. Diagnostic and therapeutic endoscopy. Curr Opin Gastroenterol. 1999; 15:504–508. PMID: 17023997.

32. Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011; 60:1327–1335. PMID: 21490373.

33. Beggs AD, Dilworth MP, Powell SL, Atherton H, Griffiths EA. A systematic review of transarterial embolization versus emergency surgery in treatment of major nonvariceal upper gastrointestinal bleeding. Clin Exp Gastroenterol. 2014; 7:93–104. PMID: 24790465.

34. Eriksson LG, Ljungdahl M, Sundbom M, Nyman R. Transcatheter arterial embolization versus surgery in the treatment of upper gastrointestinal bleeding after therapeutic endoscopy failure. J Vasc Interv Radiol. 2008; 19:1413–1418. PMID: 18755604.

35. Loffroy R, Estivalet L, Cherblanc V, et al. Transcatheter embolization as the new reference standard for endoscopically unmanageable upper gastrointestinal bleeding. World J Gastrointest Surg. 2012; 4:223–227. PMID: 23467300.

Fig. 1
Strategy algorithm for the management of suspected upper gastrointestinal (GI) bleeding. EGD, esophagogastroduodenoscopy; PPI, proton pump inhibitor; CT, computed tomography.
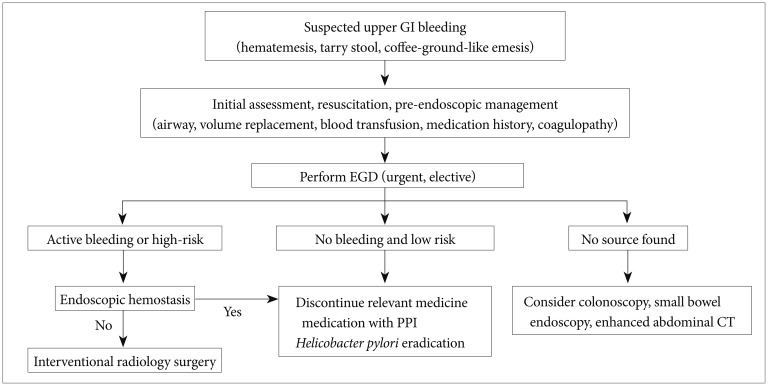
Fig. 2
Endoscopic features according to the Forrest classification. (A) Active spurting bleeding (IA). (B) Active oozing bleeding (IB). (C) No active bleeding with visible vessel (IIA). (D) Broad adherent clot (IIB). (E) Hematin-covered flat spots (IIC). (F) No bleeding stigmata with clean base ulcer (III).
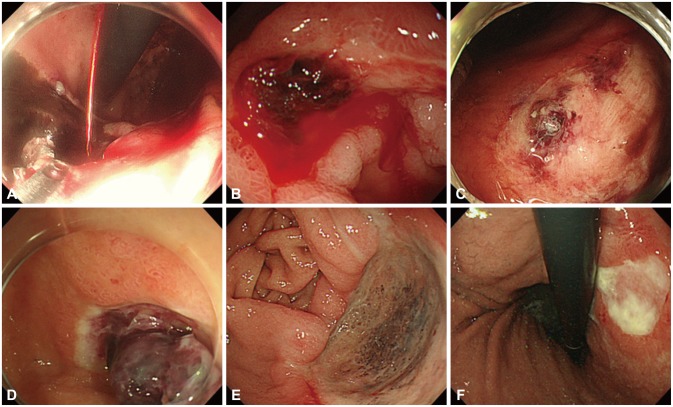
Fig. 3
Endoscopic hemostatic devices. (A) Injecting epinephrine solution into the base. (B) Mechanical clips with long arms (left) and short arms (right). (C) Clip deployment. (D) Argon plasma coagulation for active oozing from telangiectasia (square). (E) Grasping the bleeding vessel with coagulation forceps. (F) Successful burnout.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download