Abstract
Palliation of jaundice improves the general health of the patient and, therefore, surgical outcomes. Because of the complexity and location of strictures, especially proximally, drainage has been accompanied by increased morbidity due to sepsis. Another concern is the provocation of an inflammatory and fibrotic reaction around the area of stent placement. Preoperative biliary drainage with self-expanding metallic stent (SEMS) insertion can be achieved via a percutaneous method or through endoscopic retrograde cholangiopancreatography. A recently published multicenter randomized Dutch study has shown increased morbidity with preoperative biliary drainage. A Cochrane meta-analysis has also shown a significantly increased complication rate with preoperative drainage. However, few of these studies have used a SEMS, which allows better biliary drainage. No randomized controlled trials have compared preoperative deployment of SEMS versus conventional plastic stents. The outcomes of biliary drainage also depend on the location of the obstruction, namely the difficulty with proximal compared to distal strictures. Pathophysiologically, palliation of jaundice will benefit all patients awaiting surgery. However, preoperative drainage often results in increased morbidity because of procedure-related sepsis. The use of SEMS may change the outcome of preoperative biliary drainage dramatically.
Go to : 
Bile duct stricture is a challenging clinical condition that requires a coordinated multidisciplinary approach involving gastroenterologists, radiologists, and surgical specialists. These biliary strictures can be categorized according to the nature of their etiology, which can be benign or malignant. Malignant strictures are usually the result of either a primary bile duct cancer, such as a cholangiocarcinoma (CCA) causing bile duct lumen narrowing and obstructing bile flow, or extrinsic compression of bile ducts by the surrounding lymph nodes or neoplasms of adjacent organs, namely the gallbladder, pancreas, or liver. Painless obstructive jaundice is a usual manifestation of malignant biliary strictures. The most common cause of malignant distal biliary obstruction is pancreatic cancer, and 70% to 90% of these patients develop jaundice during the course of their disease.1 Because of bile flow obstruction, the presence of toxic substances can result in multiple physiological disturbances. These distorted physiologic mechanisms can result in kidney failure, cardiac dysfunction, liver injury, hemostatic abnormalities, and altered body immunity.2 Hence, palliation of jaundice improves the general health of the patient and surgical outcomes and has made this therapeutic aspect promising. We review the therapeutic options for malignant biliary obstruction and discuss the role of self-expanding metallic stents (SEMS) for preoperative biliary drainage (PBD).
Go to : 
Operative removal of cancers causing malignant biliary strictures is currently the curative treatment of choice. However, these treatments are typically major operations associated with high morbidity.2 The concept is that preoperative reduction of serum bilirubin would reduce postoperative complications. Preoperative drainage might also favorably influence sepsis, endotoxemia, and intravascular coagulation.3 Such drainage can be achieved endoscopically, surgically, or percutaneously. Endoscopic retrograde cholangiopancreatography (ERCP) with biliary drainage has become the standard practice for treating this type of obstructive jaundice. In resectable cancers, preoperative drainage should be based on multiple factors:
1) The period of time from clinical presentation to anticipated surgery. If a patient undergoes pancreaticoduodenectomy within a few days of presentation and is a candidate for surgery, no instrumentation is preferable before surgery.
2) The presence of an urgent indication for biliary drainage, namely cholangitis, severe pruritus, or severe obstruction with very high bilirubin levels.
3) The adequacy of staging prior to ERCP. In some patients, adequate staging may not be possible and a stent placement may be preferred prior to laparotomy.
4) The functional status of the patient. Many patients are in poor nutritional status because of obstructive jaundice, which could improve following PBD.
5) The plan for neoadjuvant chemoradiation for locally advanced or borderline resectable pancreatic cancer when drainage in this setting would prevent hepatotoxicity from chemotherapeutic agents.4
Go to : 
PBD for obstructive malignant biliary strictures can be achieved via a percutaneous method, ERCP, or surgical bypass. Placement of a drainage system such as stents by means of the endoscopic route gained tremendous popularity because of its advantages of being least invasive and "easier to do" with fewer complications. Despite multiple reports of increased cost effectiveness,5 higher technical success, better biliary decompression, and less recurrent cholangitis6 when compared with endoscopic drainage, the percutaneous method is still not as popular as endoscopic drainage because of its perceived disadvantages such as the risk of liver puncture-related hemorrhage, difficult-to-manage drainage system, cutaneous infection, etc. Above all, the most feared complication as reported by Takahashi et al.7 was a 5.2% percutaneous transhepatic biliary drainage catheter tract recurrence, a figure that is not negligible when considering the ensuing curative surgery. In another earlier drainage study, Speer et al.8 revealed that patients randomized to the endoscopic method had a significantly higher success rate for jaundice relief (81% vs. 61%, p=0.017) and significantly lower 30-day mortality (15% vs. 33%, p=0.016) compared to those in the percutaneous group. The higher mortality after percutaneous stenting was related to complications associated with liver puncture, which were hemorrhage and bile leaks.8
The first description of drainage via ERCP was by Cotton at the Middlesex group who subsequently performed several studies that showed superiority with respect to palliation of jaundice and decreased complications compared to percutaneous drainage and surgical bypass. In this study, the stented patients had lower procedure-related mortality (3% vs. 14%, p=0.01), lower major complication rates (11% vs. 29%, p=0.02), and shorter median total hospital stays (20 days vs. 26 days, p=0.001). Conversely, recurrent jaundice occurred in 36 stented patients and 2 surgical patients. Late gastric outlet obstruction occurred in 17% of stented patients and 7% of the surgical group. Despite the early benefits of stenting, there was no significant difference in overall survival between the two groups (median survival: surgical, 26 weeks; stented, 21 weeks; p=0.065).8,9 In a subsequent meta-analysis in 2006, Moss et al.10 came to the same conclusion, i.e., endoscopic decompression via plastic stenting carried a lower risk of complications (relative risk [RR], 0.60; 95% confidence interval [CI], 0.45 to 0.81) but a higher risk of recurrent biliary obstruction (RR, 18.59; 95% CI, 5.33 to 64.86) when compared with traditional surgical bypass.
Other areas of concern are stricture location and choice of drainage endoprostheses. For strictures that are located proximally (high-grade hilar stricture), drainage has been associated with increased morbidity to the patient, especially sepsis, most particularly with increased rates of cholangitis. The choice between a plastic or metallic stent traditionally depends on the expected survival duration in those with unresectable tumors. However, in those with resectable tumors, the preoperative waiting period seems to be the most important factor influencing the decision for PBD and subsequent choice of drainage endoprostheses. The outcomes with SEMS have been superior to plastic stenting because of the increased SEMS patency and the ability to provide rapid biliary decompression from the larger lumens.11,12,13,14 However, the concern with SEMS placement is the provocation of an inflammatory and fibrotic reaction around the area of stent placement, making subsequent surgical dissection more difficult.15,16 Placement of such stents for biliary drainage before surgery has been a subject of great debate and controversy.
Go to : 
Contrary to the aforementioned advantages of preoperative drainage, several earlier studies actually reported more drainage-related and postoperative complications in those receiving percutaneous PBD.3,17 Subsequent to these publications were several other more recent studies, chiefly the multicenter randomized Dutch study, which showed no benefit but instead increased morbidity with biliary drainage compared to proceeding directly to surgery within 7 days. The serious complication rates in this paper were 39% in the early surgery and 74% in the biliary drainage group, of which cholangitis was reported to be frequent and an important reason for readmission.18 In a study from Switzerland by Martignoni et al.19 the unwanted effect of preoperative drainage showing cumulative postoperative morbidity was 47%, the reoperation rate was 4.3%, and the mortality rate was 2.3%. Also, there was no difference in total morbidity, infectious complications, reoperation rates, mortality, or long-term survival between patients with or without PBD.19 A 2012 Cochrane meta-analysis by Fang et al.2 showed a significantly higher occurrence of serious morbidity in the PBD group compared to the direct early surgery group (RR, 1.66; 95% CI, 1.28 to 2.16; p=0.0002). There was no significant difference in the overall mortality rate in the groups. However, this study had several discrepancies and different variations in terms of heterogeneity of the studies, such as the site of obstruction, type of drainage performed, and use of only plastic stents.2
The benefits of PBD in operable tumors have also been shown. PBD is clearly indicated in the presence of cholangitis or significant hepatic dysfunction secondary to prolonged obstruction, both of which may be expected to increase perioperative complications. Early drainage is also considered if surgery is delayed for logistical reasons or to permit delivery of neoadjuvant chemoradiotherapy, particularly in patients with deep jaundice or pruritus. This was attributed to an association between hyperbilirubinemia and increased perioperative complications.20
The outcomes of biliary drainage also depend on the location of the obstruction. Distal obstruction is more amenable to effective endoscopic drainage compared to more proximal hilar tumors, which are invariably associated with higher morbidity. With regard to specific indications, in patients with hilar CCA undergoing major liver resection, drainage of the future liver remnant has been recommended to induce hypertrophy and thereby reduce the risk of postoperative liver failure.21 In a retrospective analysis by Farges et al.22 although there appeared to be no difference in overall mortality in those who underwent PBD and those who did not, a subanalysis revealed that PBD for hilar CCA was associated with a decreased mortality rate after right hepatectomy (adjusted odds ratio [OR], 0.29; 95% CI, 0.11 to 0.77; p=0.013) but an increased mortality rate after left hepatectomy (adjusted OR, 4.06; 95% CI, 1.01 to 16.30; p=0.035). The postulation was such that drainage for those who underwent right hepatectomy induced hypertrophy of the remaining left lobe and thus reduced mortality secondary to hepatic dysfunction following surgery, whereas drainage for those who underwent left hepatectomy did not gain much benefit from the right lobe hypertrophy as it was already a dominant lobe but had increased morbidity and mortality because of drainage-related infectious complications.22
Other older studies have depicted the importance of biliary drainage before tumor resection in which operative mortality was decreased from 28% to 8%.23,24 However, few of these studies analyzed the use of SEMS. SEMS have been shown to afford better biliary drainage with three times larger stent lumens, longer patency, better ease of deployment, and decreased overall complication rates, especially related to cholangitis. No prospective randomized controlled trials have compared primarily the preoperative deployment of SEMS versus direct surgery in patients with biliary obstruction.
Go to : 
SEMS have been widely used for palliation of obstructive jaundice in patients with unresectable pancreaticobiliary cancers or those with locally advanced and metastatic cancers. Nevertheless, SEMS are increasingly being recognized for use in patients with resectable cancers or benign biliary strictures. 11,12,25,26,27,28,29
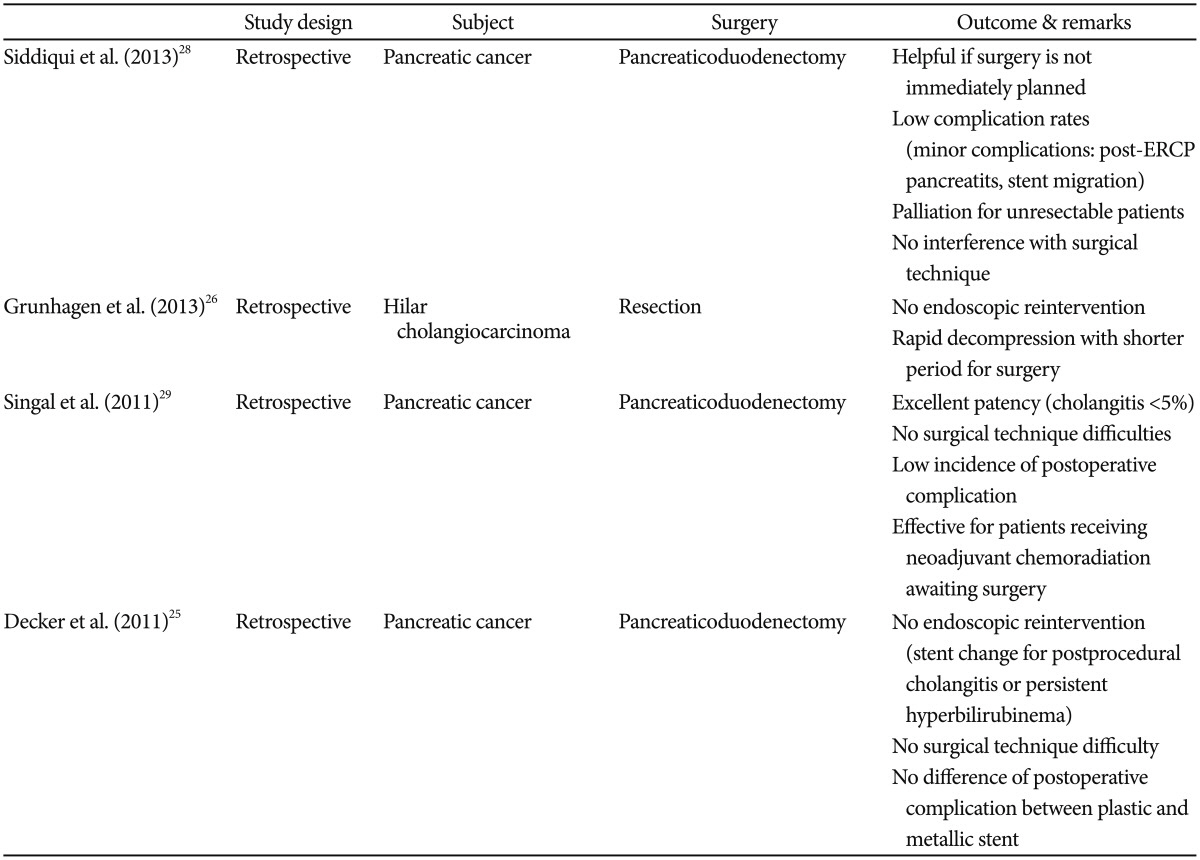
Most of these reports are retrospective studies that demonstrated the utility of SEMS for preoperative biliary decompression in patients with resectable or borderline resectable tumors. In a 5-year retrospective review of 241 pancreatic cancer cases, Siddiqui et al.28 found metallic biliary stents to be safe and effective for biliary drainage in patients with resectable cancer before undergoing pancreaticoduodenectomy and those receiving neoadjuvant therapy. They also determined the median time from stent placement to surgery in patients with operable pancreatic cancer, which was 4 weeks, a relatively long period of time for most patients to proceed without some form of biliary decompression. This potential delay was predominantly a result of medical optimization of patient comorbidities before surgery. This further reinforces the argument that metal stents should be considered for patients with resectable pancreatic cancer, especially if surgery is not immediately contemplated. Also, SEMS provide sustained palliation for patients with unresectable tumors with a low incidence of serious adverse events. A considerable number of patients never underwent definitive surgery, and SEMS in these patients served as palliative devices. During surgery, SEMS did not interfere or pose any technical difficulty. The complication rate was low at <10%, and complications were mostly minor.28 In a retrospective evaluation of operable hilar CCA, Grunhagen et al.26 revealed that SEMS provided rapid biliary decompression because of the wider lumen, making proximal sepsis less likely. Also, no endoscopic reinterventions were performed in these series, and rapid decompression enabled the patients to recover immediately; therefore, the interval between biliary decompression and explorative laparotomy was relatively short.26 In another review, Singal et al.29 showed that SEMS provided excellent patency with cholangitis occurring in <5% of cases, did not affect surgical technique, resulted in few postoperative complications, and was effective for patients receiving preoperative chemoradiation awaiting surgery. Overall, all of these studies allay the fear that endoscopically placed SEMS as part of PBD may interfere with subsequent surgeries. It is interesting to note that no special preferences in SEMS type were observed.25,28,29 These studies were, however, limited by their retrospective design and lack of a comparative group (Table 1).
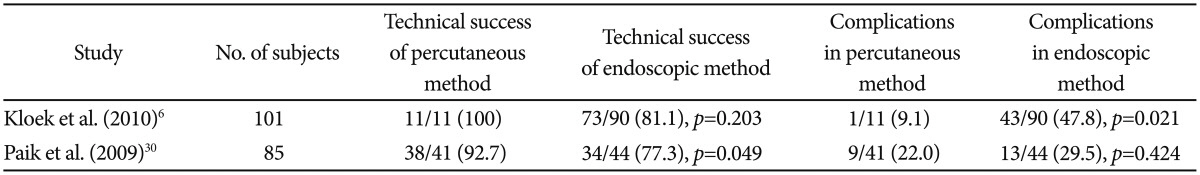
In most parts of the world, endoscopic PBD is often performed as a primary intervention and is only followed by a percutaneous method if the endoscopic method fails to achieve the required drainage. Available evidence seems to suggest that the percutaneous method with either external drainage or internal drainage with antegrade stenting is more effective. In a Dutch study published in 2010, Kloek et al.6 reported a higher technical success rate (100% vs. 81%), fewer infectious complications (9% vs. 48%, p<0.05), and fewer drainage procedures (1.4 vs. 2.8, p<0.01) in the percutaneous group versus the endoscopy group in patients with resectable hilar CCA. Published around the same time as the Dutch study was another Korean study concerning SEMS deployed by either percutaneous or endoscopic means in patients with unresectable hilar CCA. This study further affirmed that the technical success rate in the percutaneous SEMS group was higher than that in the endoscopic SEMS group (92.7% vs. 77.3%).30 However, endoscopy is still the preferred initial choice of decompression by most, as it is deemed to be less invasive and carries fewer complications such as liver puncture-related bleeding, bile peritonitis, tumor seeding, etc. PBD via the percutaneous route seems to be an effective rescue therapy when endoscopic drainage is impossible. Perhaps the percutaneous route should be considered as a primary intervention in proximal disease (high-grade hilar disease) when technical success via the endoscopy is considered to be even lower and is associated with a risk of causing further infectious complications when often the stained biliary system cannot be drained (Table 2).
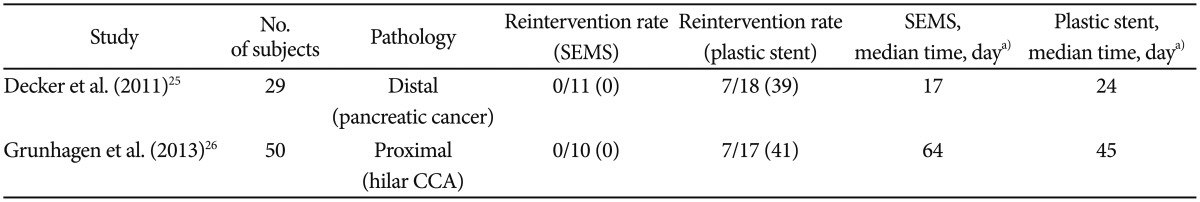
Decker et al.25 retrospectively compared the rate of endoscopic reinterventions in patients with pancreatic cancer undergoing plastic or SEMS placements for preoperative biliary decompression. The patients who underwent SEMS placement required no endoscopic reintervention before undergoing pancreaticoduodenectomy. On the contrary, nearly 40% of the patients who underwent plastic stent placement required a stent exchange because of the development of cholangitis or persistent hyperbilirubinemia. The authors also found neither technical difficulty encountered during surgery with SEMS nor a difference in the rate of postoperative complications between plastic and metallic stents.25 With a larger and slightly different cohort (proximal stricture secondary to hilar CCA) than Decker et al.,25 Grunhagen et al.26 reported similar findings of no preoperative reintervention in the group that received SEMS placement and an up to 40% reintervention rate in the group that received plastic stenting. Interestingly, such excellent findings were also seen in Grunhagen's study despite the longer median time between SEMS placement and surgery (64 days in SEMS and 45 days in plastic) compared to Decker's study (17 days in SEMS and 24 days in plastic) (Table 3).26
In 2011, Singal et al.29 reported that endoscopically placed SEMS had a relatively low incidence of reintervention (13/79, 16%) despite a long median time of 120 days before surgery as a majority of the study patients (95%) underwent neoadjuvant chemotherapy prior to surgery. In another large retrospective multicenter study (241 subjects), Siddiqui et al.28 revealed successful biliary decompression by endoscopic SEMS placement in all patients with either resectable or unresectable pancreatic cancer. The median time to surgery post-SEMS placement was 4 weeks in the direct surgery group but it was 15 weeks in those who received neoadjuvant therapy before surgery. Overall stent occlusion was seen in 14 subjects, i.e., 5.8% of the study population, throughout the study period. The exact preoperative reintervention rate was unclear in this study, as not all patients in the direct surgery or neoadjuvant group ended up with definitive surgery (Table 4).28
Grunhagen et al.26 described the utility of SEMS in proximal disease. In their retrospective study, SEMS was compared with plastic stents in hilar CCA as a means for PBD. Of the 10 patients who received SEMS, none experienced stent failure. Of the 17 patients who received plastic stents, seven (41%) had stent occlusion prior to surgery. The median time to surgery was 45 days and 68 days in patients with SEMS and plastic stents, respectively.26 It is important to note that fully covered SEMS are not indicated for proximal obstruction because they may occlude the contralateral biliary system, side branches, or the cystic duct, leading to infectious complications such as cholangitis or cholecystitis.
Go to : 
Preoperative relief of biliary obstruction and palliation of jaundice are crucial and at the same time beneficial to those patients in whom early surgery is not feasible. This generally improves health and surgical outcomes. There are still conflicting issues about preoperative drainage resulting in increased morbidity and complication rates; hence, PBD is not recommended when early surgery is planned. On the other hand, its advantages are clearly seen in situations such as cholangitis, severe hepatic dysfunction, delay in surgery, need for neoadjuvant chemotherapy, and specific indications such as patients with hilar CCA undergoing right hepatic resection. The location of the biliary obstruction is important to the technical success of drainage and should be taken into consideration when deciding on PBD and subsequently on the method of PBD and the choice of biliary endoprostheses. The utility of a better SEMS with a wider lumen may somehow change the outcome of PBD dramatically, as shown in the previously discussed studies that indicated its advantages in providing rapid biliary decompression and significant reduction in preoperative reintervention rates when compared to a plastic stent. Furthermore, it did not interfere with subsequent surgery in many studies. However, further prospective randomized controlled studies are necessary to demonstrate the efficacy of SEMS in PBD, as most of the studies presented thus far are retrospective in design.
Go to : 
Acknowledgments
This article is a write-up of a lecture presented by professor KLG at the IDEN meeting on 31 May 2014.
Go to : 
References
1. Lee JH. Self-expandable metal stents for malignant distal biliary strictures. Gastrointest Endosc Clin N Am. 2011; 21:463–480. PMID: 21684465.

2. Fang Y, Gurusamy KS, Wang Q, et al. Pre-operative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2012; 9:CD005444. PMID: 22972086.

3. Hatfield AR, Tobias R, Terblanche J, et al. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet. 1982; 2:896–899. PMID: 6126752.
4. Jaganmohan S, Lee JH. Self-expandable metal stents in malignant biliary obstruction. Expert Rev Gastroenterol Hepatol. 2012; 6:105–114. PMID: 22149586.

5. Hong SK, Jang JY, Kang MJ, Han IW, Kim SW. Comparison of clinical outcome and cost-effectiveness after various preoperative biliary drainage methods in periampullary cancer with obstructive jaundice. J Korean Med Sci. 2012; 27:356–362. PMID: 22468097.

6. Kloek JJ, van der Gaag NA, Aziz Y, et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg. 2010; 14:119–125. PMID: 19756881.

7. Takahashi Y, Nagino M, Nishio H, Ebata T, Igami T, Nimura Y. Percutaneous transhepatic biliary drainage catheter tract recurrence in cholangiocarcinoma. Br J Surg. 2010; 97:1860–1866. PMID: 20799295.

8. Speer AG, Cotton PB, Russell RC, et al. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987; 2:57–62. PMID: 2439854.

9. Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994; 344:1655–1660. PMID: 7996958.
10. Moss AC, Morris E, Leyden J, MacMathuna P. Malignant distal biliary obstruction: a systematic review and meta-analysis of endoscopic and surgical bypass results. Cancer Treat Rev. 2007; 33:213–221. PMID: 17157990.

11. Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992; 340:1488–1492. PMID: 1281903.

12. Kaassis M, Boyer J, Dumas R, et al. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003; 57:178–182. PMID: 12556780.

13. Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006; 63:986–995. PMID: 16733114.

14. Yeoh KG, Zimmerman MJ, Cunningham JT, Cotton PB. Comparative costs of metal versus plastic biliary stent strategies for malignant obstructive jaundice by decision analysis. Gastrointest Endosc. 1999; 49(4 Pt 1):466–471. PMID: 10202060.

15. Ayaru L, Kurzawinski TR, Shankar A, Webster GJ, Hatfield AR, Pereira SP. Complications and diagnostic difficulties arising from biliary self-expanding metal stent insertion before definitive histological diagnosis. J Gastroenterol Hepatol. 2008; 23:315–320. PMID: 18289360.

16. Lytras D, Olde Damink SW, Amin Z, Imber CJ, Malagó M. Radical surgery in the presence of biliary metallic stents: revising the palliative scenario. J Gastrointest Surg. 2011; 15:489–495. PMID: 21246414.

17. McPherson GA, Benjamin IS, Hodgson HJ, Bowley NB, Allison DJ, Blumgart LH. Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg. 1984; 71:371–375. PMID: 6372935.

18. van der Gaag NA, Rauws EA, van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010; 362:129–137. PMID: 20071702.

19. Martignoni ME, Wagner M, Krähenbühl L, Redaelli CA, Friess H, Büchler MW. Effect of preoperative biliary drainage on surgical outcome after pancreatoduodenectomy. Am J Surg. 2001; 181:52–59. PMID: 11248177.

20. Coss A, Byrne MF. Preoperative biliary drainage in malignant obstruction: indications, techniques, and the debate over risk. Curr Gastroenterol Rep. 2009; 11:145–149. PMID: 19281702.

21. Belghiti J, Ogata S. Preoperative optimization of the liver for resection in patients with hilar cholangiocarcinoma. HPB (Oxford). 2005; 7:252–253. PMID: 18333201.

22. Farges O, Regimbeau JM, Fuks D, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013; 100:274–283. PMID: 23124720.

23. Nakayama T, Ikeda A, Okuda K. Percutaneous transhepatic drainage of the biliary tract: technique and results in 104 cases. Gastroenterology. 1978; 74:554–559. PMID: 415929.
24. Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg. 1935; 102:763–779. PMID: 17856666.
25. Decker C, Christein JD, Phadnis MA, Wilcox CM, Varadarajulu S. Biliary metal stents are superior to plastic stents for preoperative biliary decompression in pancreatic cancer. Surg Endosc. 2011; 25:2364–2367. PMID: 21373939.

26. Grunhagen DJ, Dunne DF, Sturgess RP, et al. Metal stents: a bridge to surgery in hilar cholangiocarcinoma. HPB (Oxford). 2013; 15:372–378. PMID: 23458664.
27. Isayama H, Komatsu Y, Tsujino T, et al. A prospective randomised study of "covered" versus "uncovered" diamond stents for the management of distal malignant biliary obstruction. Gut. 2004; 53:729–734. PMID: 15082593.

28. Siddiqui AA, Mehendiratta V, Loren D, et al. Self-expanding metal stents (SEMS) for preoperative biliary decompression in patients with resectable and borderline-resectable pancreatic cancer: outcomes in 241 patients. Dig Dis Sci. 2013; 58:1744–1750. PMID: 23179157.

29. Singal AK, Ross WA, Guturu P, et al. Self-expanding metal stents for biliary drainage in patients with resectable pancreatic cancer: single-center experience with 79 cases. Dig Dis Sci. 2011; 56:3678–3684. PMID: 21750930.

30. Paik WH, Park YS, Hwang JH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. 2009; 69:55–62. PMID: 18657806.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download