Abstract
Eosinophilic gastroenteritis is very rare disorder that is characterized by eosinophilic infiltration of the gastrointestinal tract in the absence of any definite causes of eosinophilia. It is associated with various clinical gastrointestinal manifestations, and depends on the involved layer and site. We report a case of eosinophilic gastritis presenting with severe necrosis. The symptoms disappeared immediately after beginning steroid treatment, and the eosinophil count decreased to the reference range. The patient showed eosinophilic gastritis characterized by necrotic change such as necrotizing gastritis. It is a unique presentation of eosinophilic gastritis. To the best of our knowledge, no case of eosinophilic gastritis characterized by necrotic change such as necrotizing gastritis has been previously reported in Korea.
Eosinophilic gastritis is an extremely rare disease that is characterized by eosinophilic infiltration of the various layers of the gastrointestinal tract in the absence of any definite causes of eosinophilia. Patients with eosinophilic gastritis have diverse symptoms, including abdominal pain, emesis, abdominal distension, and weight loss. These symptoms are associated with eosinophilic infiltration of the various layers of the gastrointestinal tract. Endoscopy and histopathological evaluations are a critical part of the diagnostic process. The disease has to be distinguished from generalized eosinophilic disorder presenting with involvement of other organs. Steroid is the cornerstone of treatment. The prognosis of the disease has been not clearly investigated.
A 52-year-old female patient visited another hospital because of epigastric pain and tenderness. She was not taking any medicines, including herbs and drugs. She had no history of allergic diseases such as eczema or atopy, or food or drug allergies. She also had no family history of eczema or atopy.
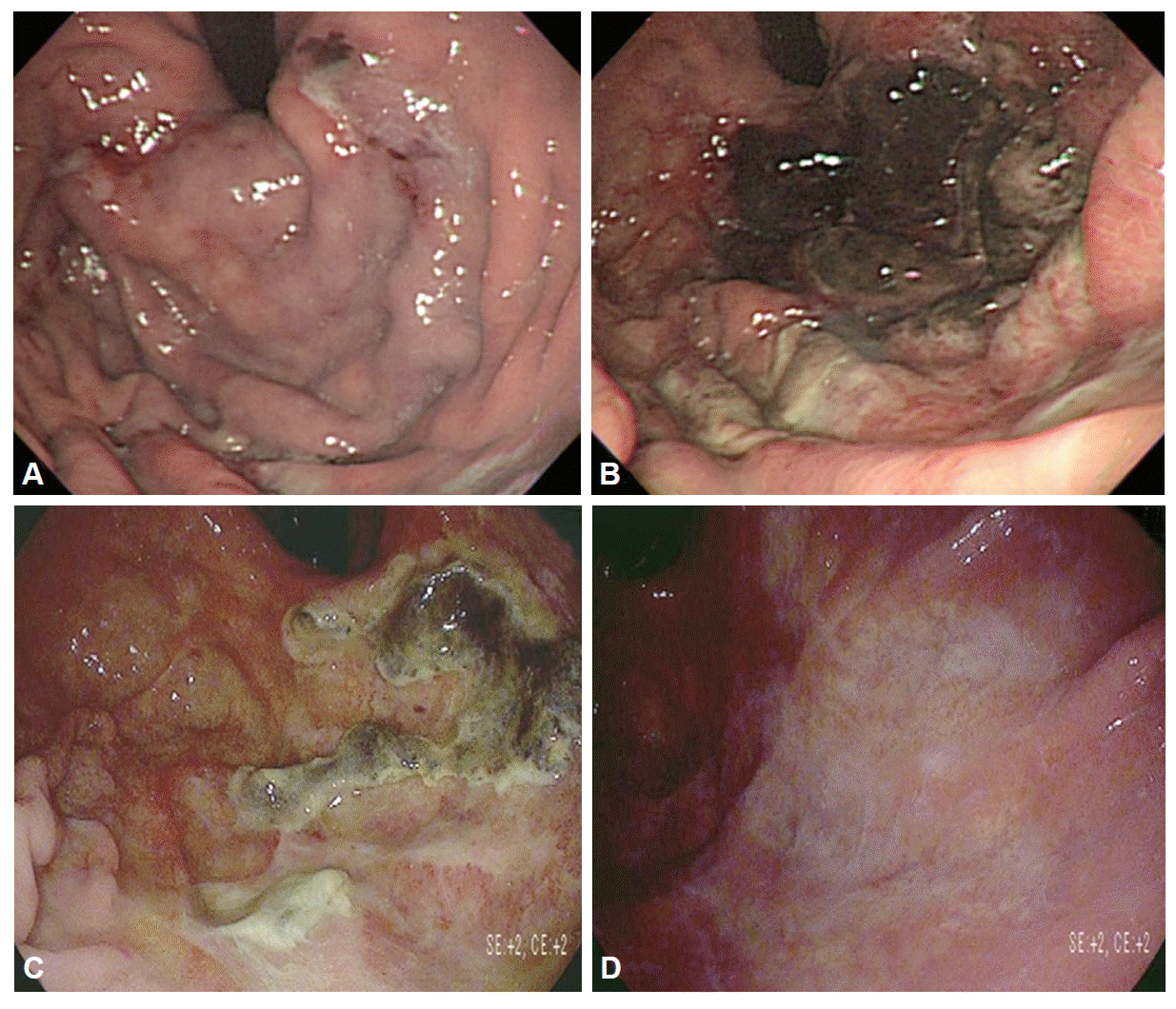
She presented with epigastric pain and tenderness. At the time of the initial presentation, she had undergone esophagogastroduodenoscopy (EGD), which revealed multiple focal ulcerative lesions with diffuse discoloration and edematous change of the rugae in the gastric fundus, cardia, and upper body (Fig. 1A). The symptoms had been recalcitrant to treatment with proton pump inhibitors, fasting, and fluid. She was referred to our hospital for further evaluation and treatment. She had reported no nausea, vomiting, hematemesis, or melena.
On admission, her blood pressure was 110/60 mm Hg, pulse rate 76 beats per minute, respiratory rate 22 breaths per minute, and body temperature 36.2℃. Physical examination revealed tenderness of the epigastric area. Her white blood cell count was 22,770/mm3 with markedly increased eosinophils (5,009/mm3, 22%). The C-reactive protein concentration was 13.95 mg/dL. Her other blood chemistry test results were normal. The tests for viral markers included hepatitis A, B, and C virus and human immunodeficiency virus; those for autoimmune antibodies included anti-nuclear, anti-double stranded DNA, and anti-neutrophilic cytoplasmic antibodies; and those for tumor markers included α-fetoprotein, carbohydrate antigen 19-9, and carcinoembryonic antigen. The prothrombin time and partial thromboplastin time were normal. No pathogens were detected in the microbiological blood, urine, and sputum cultures.
The total immunoglobulin E (IgE) concentration was 61.7 kU/L (reference, 0 to 200). The concentration of antigen-specific IgE to the Anisakis simplex was 0.57 kU/L (class 1). The serological test for Toxocara antibodies (IgG) was positive, whereas those for Echinococcus, Paragonimus westermani, Sparganumi, and Trichinella antibodies were negative. Subsequent evaluation with stool analysis showed no evidence of ova and parasites. The chest and abdomen radiographic examinations were normal.
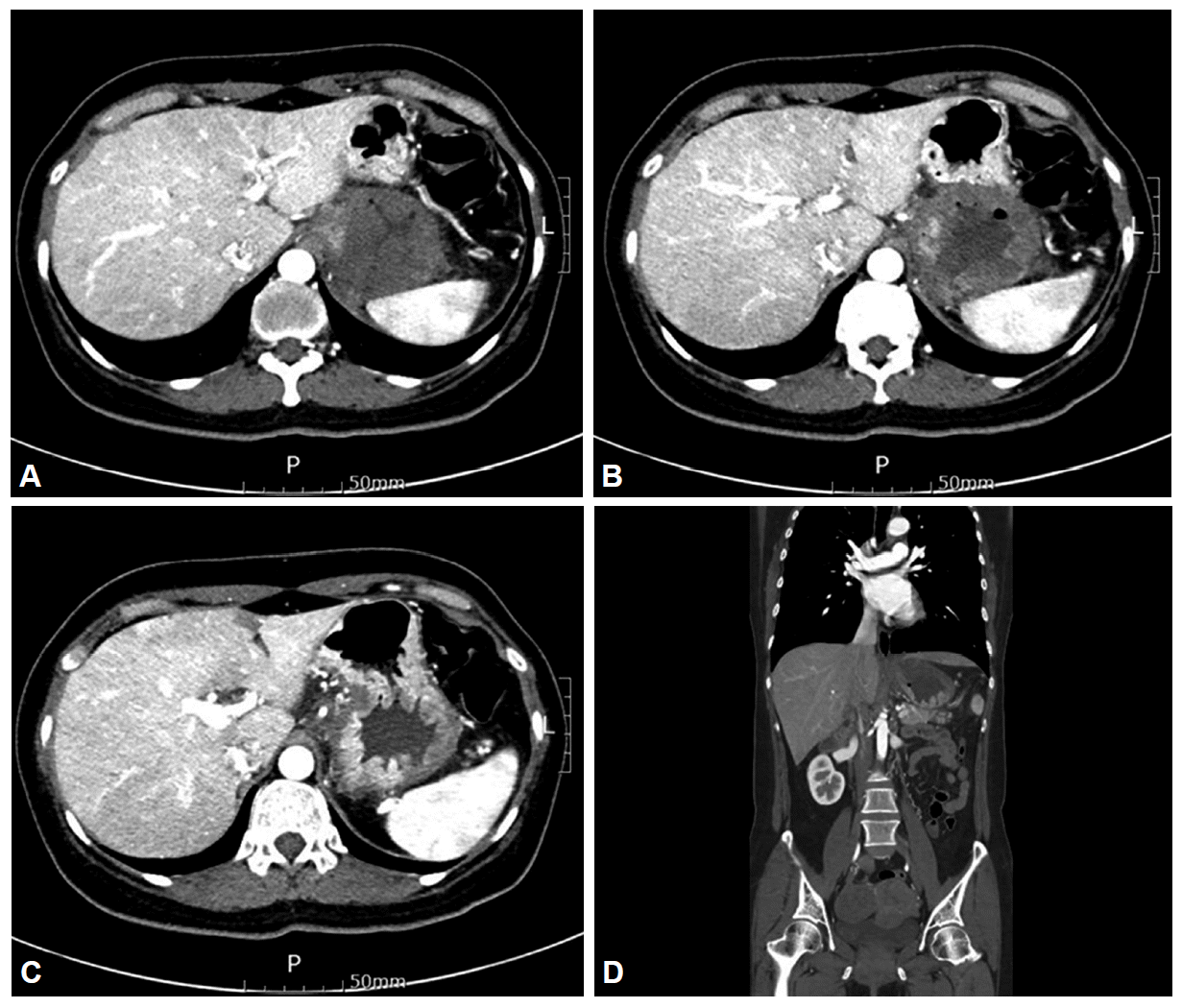
To investigate the cause of the epigastric pain and tenderness, abdominal computed tomography was performed, which revealed severe edematous wall thickening with focal localized low attenuation of the fundus and cardia of the stomach (Fig. 2).
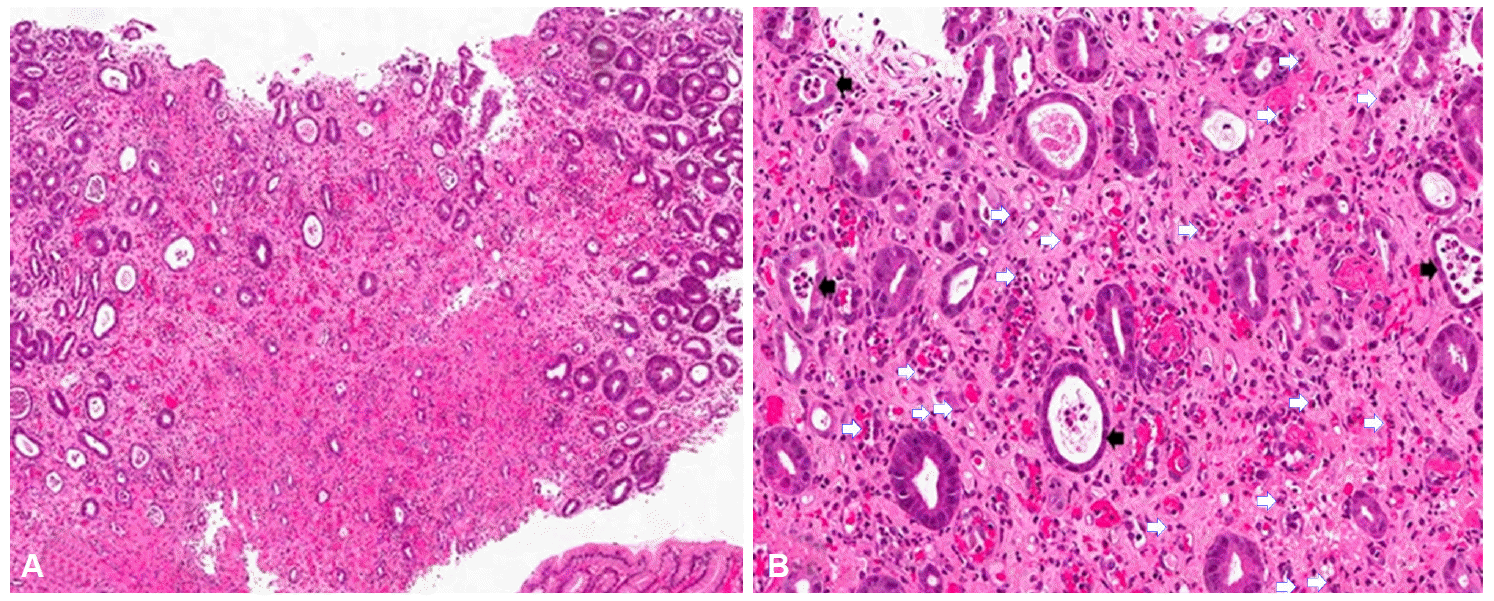
She underwent a repeated EGD, which showed diffuse necrotic change in the fundus, cardia, and upper body (Fig. 1B). A biopsy specimen was obtained during EGD; a rapid urease test (CLOtest) revealed no evidence of Helicobacter pylori. On histopathologic evaluation, the gastric surface was found to be eroded and the underlying lamina propria showed dense eosinophilic infiltration. The gastric glands were atrophied and frequently showed crypt abscesses. The histopathologic result was highly suggestive of eosinophilic gastritis (Fig. 3).
She was treated with empirical intravenous antibiotics (cefoperazone and metronidazole) immediately after her transfer because infectious gastritis was not ruled out. Although she was treated with a broad-spectrum anthelmintic (albendazole), antibiotics, proton pump inhibitor, and prokinetic agent during the next 5 days, her symptoms became worse. Eosinophilic gastritis was diagnosed according to the clinical pictures, laboratory findings, and endoscopic findings. She was immediately started on methylprednisolone (62.5 mg/day; this dose was maintained for 7 days). The symptoms disappeared just after beginning steroid treatment. After 7 days of steroid treatment, the eosinophil count decreased to 7.1%. It was important to judge the necessity of surgical treatment at that time. Therefore, she underwent a repeated short-term follow-up EGD that showed regenerative epithelial tissue with peeling off, of the necrotic tissue (Fig. 1C). The treatment was switched to 30 mg/day prednisone for 1 month. She underwent a repeated EGD that showed the replacement of white color scar tissue (Fig. 1D). The eosinophil count decreased to 2.9% with an absolute eosinophil count of 437/mm3.
Currently, she is being treated with 30 mg/day prednisone and is showing considerable clinical improvement.
Eosinophilic gastroenteritis is an uncommon and rarely reported disorder characterized by eosinophilic infiltration of the various tissue layers of the digestive tract in the absence of any definite causes of eosinophilia, without eosinophilic infiltration in other organs. The diagnosis of eosinophilic gastroenteritis is based on the following criteria: the presence of gastrointestinal symptoms, histological presentation of eosinophilic infiltration in tissue layers of the digestive tract, presence of high eosinophil count in ascites, and no evidence of parasitic infection or eosinophilic involvement of extraintestinal organs [1]. Eosinophilic gastroenteritis can involve any portion of the gastrointestinal tract from the esophagus to the rectum, and the stomach is the most commonly involved organ, especially the antrum [2,3]. In eosinophilic gastritis, the endoscopic features are rather extensive: rugal fold thickening, erythema, friability, nodularity, gastric outlet obstruction, gastric ulcer, and even a normal mucosa [4,5]. However, necrotic change such as necrotizing gastritis is an extremely rare demonstration.
Although not clearly defined, the common pathophysiological mechanism of this disease is associated with eosinophilic infiltration and degranulation in specific tissue layers of the digestive tract [6]. This eosinophilic recruitment and activation regulated by diverse cytokines is a part of the host immune mechanism in the gastrointestinal mucosa; however, it can be a type of serious allergic or inflammatory reaction in the deeper tissue layers of the gastrointestinal tract.
Although there is no treatment consensus on eosinophilic gastroenteritis, steroid therapy is a cornerstone of treatment. An effective alleviation of symptoms usually occurs within 2 weeks. Several studies report good results with steroids in dosages from 20 to 40 mg/day, for 6 to 8 weeks [7-10]. In some case studies, leukotriene modifiers such as montelukast or mast cell stabilizers such as sodium cromoglycate have been proposed to be helpful for symptomatic improvement. Antihistamines such as ketotifen or immunosuppressants such as mycophenolate mofetil are also used in the treatment of eosinophilic gastroenteritis; however, their therapeutic effects are not clear and would require more studies [11,12].
Although rare, eosinophilic gastritis should be considered in the differential diagnosis of patients with gastrointestinal symptoms and peripheral blood eosinophilia. A number of diseases can show similarities to eosinophilic gastritis. Lymphoma of the stomach, gastric cancer, and Crohn disease involving the stomach may demonstrate endoscopic features similar to those of eosinophilic gastritis.
Gastrointestinal parasitic infection should be considered in patients with abdominal discomfort, weight loss, and peripheral eosinophilia. In particular, infestations by hookworms, Ascaris, Strongyloides, Toxocara, Trichuris, and intestinal Capillaria should be considered in patients from endemic areas.
In this case, the concentration of the antigen-specific IgE to the A. simplex was 0.57 kU/L (class 1). The mean of class 1 was weakly positive. Moreover, the serology for Toxocara antibodies (IgG) was positive. However, this is not clinically significant in Korea because Koreans often consume raw fish, which causes repeated exposures to Anisakis. In addition, she had not had contact with any animals, including dogs and cats, at least for several years. Moreover, she was treated with albendazole for 5 days, and her symptoms had become worse. After steroid treatment, the symptoms disappeared and the eosinophil count decreased to the reference range. Moreover, the follow-up EGD showed regenerative epithelial tissue with peeling off, of the necrotic tissue.
She had undergone several EGDs that showed diffuse necrotic change in the fundus, cardia, and upper body. Gangrene of the stomach is a rare and fatal condition. It begins as phlegmonous gastritis, and then progresses to the lethal severe form. The etiology includes thromboembolism and occlusion of major arterial supply, ingestion of corrosive agents, volvulus of the stomach, endoscopic hemostatic injections, and infectious gastritis [13]. In this case, there was no history suggestive of atherosclerosis, herniation, and volvulus. There was also no history of ingestion of caustic substances. Thus, the possible cause of gangrene could be infection, and she was treated with empirical intravenous antibiotics (cefoperazone and metronidazole). However, she was treated with broad-spectrum antibiotics during the next 5 days, and her symptoms became worse. The clinical pictures and laboratory findings of this patient were not compatible with infection.
The necrotic portion of the gastric high body is very vulnerable site of retching injury. This retching injury is called prolapse gastropathy syndrome, a clinical syndrome involving the invagination of part of the gastric mucosa into the lower esophagus. This syndrome occurs in patients with prolonged retching and vomiting. Direct trauma to the mucosa occurs when the gastric mucosa becomes incarcerated through the lower esophageal sphincter [14,15]. Biopsy of the affected mucosa often shows mucosal inflammation. However, this patient had no symptoms similar to prolonged retching. In addition, the endoscopic findings and histopathologic results were not compatible to prolapse gastropathy syndrome.
On the basis of the clinical picture, laboratory findings, and therapeutic results, we concluded the diagnosis of eosinophilic gastritis presenting as necrotizing gastritis in our patient. Eosinophilic gastritis presenting as a necrosis has not been previously reported in Korea. This case highlights the reality of eosinophilic gastritis presenting as necrotizing gastritis, and that endoscopy and histopathological examination of the biopsies are the most useful tools for the diagnosis of eosinophilic gastritis presenting as necrotizing gastritis. Eosinophilic gastritis should be considered in the differential diagnosis in patients with necrotic gastritis who do not respond to empirical treatment.
REFERENCES
1. Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004; 113:11–28.

2. Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990; 31:54–58.

3. Yan BM, Shaffer EA. Primary eosinophilic disorders of the gastrointestinal tract. Gut. 2009; 58:721–732.

4. Navab F, Kleinman MS, Algazy K, Schenk E, Turner MD. Endoscopic diagnosis of eosinophilic gastritis. Gastrointest Endosc. 1972; 19:67–69.

5. Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol. 1996; 91:1679.
6. Hogan SP, Rothenberg ME. Eosinophil function in eosinophil-associated gastrointestinal disorders. Curr Allergy Asthma Rep. 2006; 6:65–71.

7. Siewert E, Lammert F, Koppitz P, Schmidt T, Matern S. Eosinophilic gastroenteritis with severe protein-losing enteropathy: successful treatment with budesonide. Dig Liver Dis. 2006; 38:55–59.

8. Baig MA, Qadir A, Rasheed J. A review of eosinophilic gastroenteritis. J Natl Med Assoc. 2006; 98:1616–1619.
9. Liacouras CA, Wenner WJ, Brown K, Ruchelli E. Primary eosinophilic esophagitis in children: successful treatment with oral corticosteroids. J Pediatr Gastroenterol Nutr. 1998; 26:380–385.

11. Neustrom MR, Friesen C. Treatment of eosinophilic gastroenteritis with montelukast. J Allergy Clin Immunol. 1999; 104(2 Pt 1):506.

12. Suzuki J, Kawasaki Y, Nozawa R, et al. Oral disodium cromoglycate and ketotifen for a patient with eosinophilic gastroenteritis, food allergy and protein-losing enteropathy. Asian Pac J Allergy Immunol. 2003; 21:193–197.
13. Strauss RJ, Friedman M, Platt N, Gassner W, Wise L. Gangrene of the stomach: a case of acute necrotizing gastritis. Am J Surg. 1978; 135:253–257.

Fig. 1.
(A) The first esophagogastroduodenoscopy (EGD) revealing multiple focal ulcerative lesions with diffuse discoloration and edematous change of the rugae in the gastric fundus, cardia, and upper body. (B) The second EGD showing diffuse necrotic change in the fundus, cardia, and upper body. (C) After 7 days of steroid treatment, the third EGD showing regenerative epithelial tissue with peeling off, of the necrotic tissue. (D) The fourth EGD showing the replacement of white scar tissue.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download