Abstract
Narrow-band imaging (NBI) is a new imaging technology that was developed in 2006 and has since spread worldwide. Because of its convenience, NBI has been replacing the role of chromoendoscopy. Here we review the efficacy of NBI with/without magnification for detection, characterization, and management of colorectal polyps, and future perspectives for the technology, including education. Recent studies have shown that the next-generation NBI system can detect significantly more colonic polyps than white light imaging, suggesting that NBI may become the modality of choice from the beginning of screening. The capillary pattern revealed by NBI, and the NBI International Colorectal Endoscopic classification are helpful for prediction of histology and for estimating the depth of invasion of colorectal cancer. However, NBI with magnifying colonoscopy is not superior to magnifying chromoendoscopy for estimation of invasion depth. Currently, therefore, chromoendoscopy should also be performed additionally if deep submucosal invasive cancer is suspected. If endoscopists become able to accurately estimate colorectal polyp pathology using NBI, this will allow adenomatous polyps to be resected and discarded; thus, reducing both the risk of polypectomy and costs. In order to achieve this goal, a suitable system for education and training in in vivo diagnostics will be necessary.
Narrow-band imaging (NBI) is a method of image enhancement that modifies white light (WL) into narrowed bands of light with a center wavelength of 415 (blue) and 540 nm (green), which are absorbed by hemoglobin [1-3]. Diagnosis based on angiogenesis might be ideal for early detection and diagnosis of neoplastic lesions, because angiogenesis is essential for the transition of a premalignant lesion in a hyperproliferative state to a malignant lesion [4-6]. NBI becomes more powerful when adapted to a magnifying endoscope providing a range of low to high optical magnification (×80 maximum) by means of a simple, one-touch operation [7]. NBI providing enhanced images of vessels and surface patterns of lesions has greatly contributed to detection and diagnosis of colorectal tumors, and changed the role of ordinary diagnostic methods such as WL imaging and chromoendoscopy.
This review highlights the application of NBI colonoscopy with or without optical zoom magnification for the detection and diagnosis of colorectal lesions, and discusses the education and training of practitioners, and future perspectives.
So far, screening colonoscopy for detection of colorectal polyps has been performed using WL. With the development and dissemination of NBI, a number of randomized controlled trials for evaluating the efficacy of NBI in screening colonoscopy have been conducted, and meta-analysis of these trials has concluded that high-definition (HD) NBI colonoscopy is better than standard-definition WL colonoscopy and equal to HD-WL colonoscopy for detection of colorectal adenomas and polyps [8,9]. Therefore, at present, screening colonoscopy is performed using HD-WL, and the NBI technique is employed mainly for diagnosis of any colorectal polyps that are detected.
The brightness and resolution of colonoscopic views obtained using NBI have been improved markedly since the introduction of the Olympus 290 series (EVIS LUCERA ELITE; Olympus, Tokyo, Japan) and 190 series (EVIS EXERA III) endoscopy systems, in comparison with the previous 260 and 180 series, respectively (Figs. 1, 2). In a prospective multicenter randomized controlled trial using the 290 series, Horimatsu et al. [10] compared the mean number of colorectal polyps detected per patient between HD-NBI and HD-WL colonoscopy, and found that the number was significantly higher for the former than for the latter (2.01 vs. 1.56, p=0.032). Therefore, they concluded that HD-NBI colonoscopy using the 290 series endoscopy system was superior to HD-WL colonoscopy for detection of colorectal polyps. Moreover, they found that significantly more sessile serrated adenomas/polyps, which have recently attracted much attention as possible precursor lesions of colorectal cancers with microsatellite instability, were detected using NBI than by WL (0.05 vs. 0.01 per patient, p=0.036). Leung et al. [11] conducted a prospective randomized controlled trial to compare the adenoma and polyp detection rates between HD-NBI and HD-WL colonoscopy using the novel 190 series endoscopy system, and found that both rates were significantly higher for NBI than for WL (adenoma: 48.3% vs. 34.4%, p=0.01; polyps: 61.1% vs. 48.3%, p=0.02). Therefore, they concluded that HD-NBI colonoscopy using the 190 series was superior to HD-WL colonoscopy for detection of colorectal adenomas and polyps.
Previous studies have demonstrated the usefulness of NBI in screening endoscopy for early detection of cancers in the head and neck region and esophagus [12]. Although further studies are required to confirm the usefulness of NBI in screening colonoscopy for detection of colorectal polyps, the available evidence suggests that screening endoscopy using NBI with these newly developed endoscopy systems may become a standard tool, not only for the head and neck region and esophagus but also for the colon and rectum (Fig. 3).
In Japan, a combination of magnifying colonoscopy and chromoendoscopy has been one of the most reliable methods for distinguishing non-neoplastic from neoplastic lesions. The reported overall diagnostic accuracy of magnifying chromoendoscopy using indigo carmine is 95.6%, being 10% and 5% more superior to conventional endoscopy and chromoendoscopy, respectively [13]. Unfortunately, magnifying chromendoscopy has not disseminated around the world because magnifying endoscopy is difficult to use and chromoendoscopy is time-consuming. NBI was developed in 2006, and since then has spread and changed the conventional approach to diagnosis.
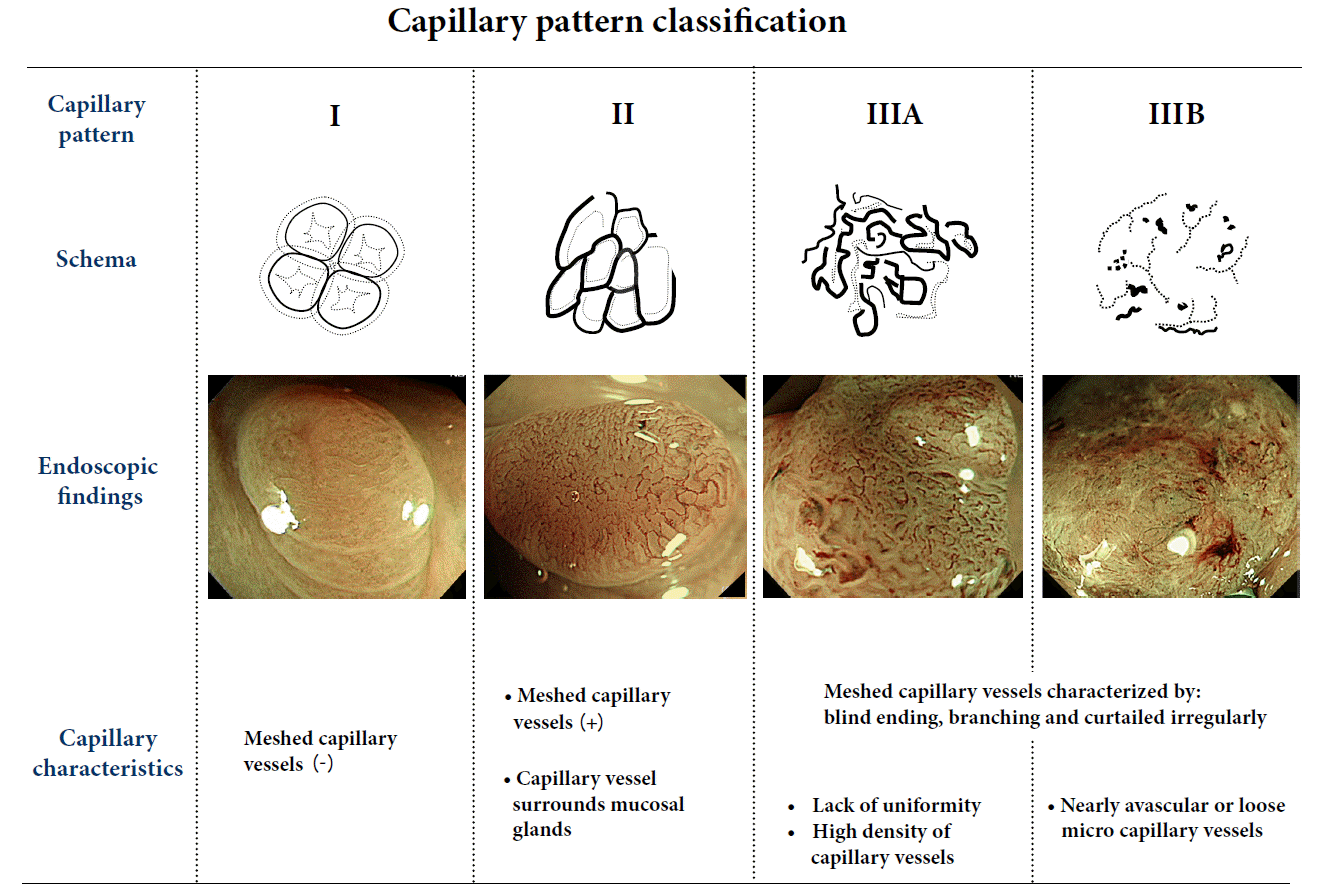
NBI allows endoscopists to recognize neoplastic lesions by emphasizing neoplastic angiogenesis. The normal mucosa consists of intestinal crypts surrounded by regular networks of capillaries, and this surface microvascular architecture (capillary pattern [CP]) can be demonstrated by NBI with magnifying colonoscopy. Vascular changes (brownish dilated and meshed vessels) are recognizable in neoplastic lesions, and are referred to as meshed capillary (MC) vessels. On the basis of these MC vessels, CP has been classified into three types (types I, II, and III) for qualitative assessment (Fig. 4) [14-16]. The presence of MC vessels is useful for differentiating small colorectal non-neoplastic from neoplastic polyps (CP type I vs. type II; accuracy 95.3%, sensitivity 96.4%, specificity 92.3%) [15]. Moreover, low-grade dysplasia can be significantly differentiated from high-grade dysplasia/invasive cancer by observing the structure of MC vessels closely (CP type II vs. type III; accuracy 95.5%, sensitivity 90.3%, specificity 97.1%) [16].
Only NBI with magnifying colonoscopy, and not chromoendoscopy, is used to distinguish neoplastic and non-neoplastic polyps during routine colonoscopy because magnifying colonoscopy with NBI is convenient to use and as accurate as magnifying colonoscopy.
NBI with magnifying colonoscopy is also useful for estimating the depth of early colorectal cancer. CP type Ⅲ lesions are subclassified into type IIIA and IIIB. This subclassification is effective for estimating the depth of cancer invasion. Type IIIB lesions are nearly avascular or have loose capillaries, and these characteristics are predictive of SM deep cancers. Ikematsu et al. [17] have reported that the accuracy, sensitivity, and specificity of CP type IIIB for differentiating SM 2-3 from M or SM1 lesions are 87.7%, 84.8%, and 88.7%, respectively. In comparison with the pit pattern classification (accuracy 98.8%, sensitivity 85.6%, specificity 99.4%) [18], the sensitivity is similar, but accuracy and specificity are markedly lower.This suggests the need for additional chromoendoscopy when a lesion is suspected to be deep submucosal invasive cancer.
NBI without magnification also provides high accuracy for differentiating between neoplastic and non-neoplastic polyps when diagnosed with high confidence (≥90% confidence for prediction of histology). Rex [19] has reported that endoscopic prediction of adenomas and hyperplastic polyps using NBI without magnification is correct in 91% and 95% of cases when the diagnosis is made with high confidence. Ignjatovic et al. [20] has also indicated that the accuracy of differentiating between neoplasic and non-neoplastic polyps is 95% for experts and 87% for non-experts when optical diagnosis can be made with high confidence. NBI without magnification is useful when diagnosis can be made with high confidence. Because magnifying endoscopy is not available outside Japan, simple classification systems for NBI with/without magnifying endoscopy were necessary in order for the system to be widely used in clinical practice. Therefore in 2011, the NBI International Colorectal Endoscopic (NICE) classification was proposed by an international group (Colon Tumor NBI Interest Group [CTNIG]), which included Yasushi Sano (Japan), Shinji Tanaka (Japan), Douglas K. Rex (U.S.), Roy M. Soetikno (U.S.), Thierry Ponchon (France), and Brian P. Saunders (UK).
The NICE classification is based on three criteria: (1) color, (2) vessels, and (3) surface pattern, and differentiates colonic lesions into three categories (Table 1). Several validation studies of the NICE classification have already been conducted. Hewett et al. [21] have reported that NBI-experienced colonoscopists were able to differentiate hyperplastic and adenomatous polyps with high confidence for 75% of small colorectal polyps by using the NICE classification (NICE1 and NICE2), and with 89% accuracy, 98% sensitivity, and 95% negative predictive value. In terms of NICE2 and NICE3, Hayashi et al. [22] have confirmed that when any one of the three criteria (color, vessels, surface pattern) of deeply SM-invasive carcinoma was present, the sensitivity was 94.9%, and the negative predictive value was 95.9%.
The NICE classification is a valid tool for differentiating not only non-neoplastic from neoplastic polyps, but also SM-invasive carcinomas from early colorectal carcinomas. However, further multicenter research involving endoscopists with different abilities will be necessary to validate these results in order to ensure that the classification can be used widely with satisfactory availability and reliability.
As mentioned above, although NBI with magnifying colonoscopy can replace magnifying chromoendoscopy for prediction of histology, it is not superior to magnifying chromoendoscopy for estimation of cancer invasion depth. Based on the available evidence, we have proposed a three-step strategy for management of colorectal lesions, which is based on NBI colonoscopy (Fig. 5) [23]. Chromoendoscopy is necessary when we suspect that a lesion has invaded deeply into the submucosal layer, a situation observed in only 5% of all neoplastic lesions.
Diagnosis of colorectal lesions by experts using NBI has been established, and the next challenge is to focus on training system for non-experts.
Recently, many articles have described the learning curve of NBI-based diagnosis using various training modules for differentiating between neoplasic and non-neoplastic lesion. Some in vitro studies have demonstrated that non-experts can achieve a high accuracy (81% to 90.8%) and good interobserver agreement with a short and simple training course or a computer-based program [24-27]. However, two prospective in vivo studies have demonstrated inferior accuracy (75.6% to 81%) after a video-based training program [28,29]. These differences suggest that assessment of endoscopic images in real-time requires expertise. Endoscopic imaging in real time can be influenced by the location of a lesion, and the presence of mucus or residual fecal material on a lesion. It is necessary to learn how to obtain good evaluable images and to evaluate lesions in vivo. Dai et al. [30] reported that a short interactive training program in combination with continual feedback involving 30 cases in vivo allowed less experienced endoscopists to improve their diagnostic accuracy for non-target lesions (70.0% to 96.7%) and target lesions (76.7% to 95.0%). Continuous feedback in vivo may be a key factor for improvement of accuracy when attempting to predict polyp histology in real time. Further investigations will be necessary to establish adequate in vivo training programs.
At present, all polyps are basically removed and submitted for formal pathological examination because conventional WL colonoscopy has limited accuracy (59% to 84%) for differentiating between neoplastic and non-neoplastic lesions [13,31-35]. If it were possible to accurately assess colorectal polyp pathology using endoscopy alone, then tiny rectosigmoid hyperplastic polyps would be left in situ, thus reducing the risks associated with polypectomy, whereas diminutive adenomas could be resected and discarded, thus saving the cost of histological evaluation. Today many endoscopic modalities, such as NBI, autofluence imaging, Fuji Intelligent Chromo Endoscopy, and i-scan are available. Which modality should be used for assessment of colorectal polyp pathology endoscopically? The American Society for Gastrointestinal Endoscopy (ASGE) has suggested that the necessary thresholds for assessment of histology using endoscopic technology are >90% agreement for determining post-polypectomy surveillance intervals with a ≥90% negative predictive value (when used with high confidence) for rectosigmoid polyps with an adenomatous histology [36]. A systematic review and meta-analysis by the ASGE has confirmed that the thresholds for real-time endoscopic assessment of the histology of diminutive polyps have been met, at least for NBI performed by an expert when assessments are made with high confidence [37]. Iwatate et al. [38] have reported that high-magnification endoscopy significantly improved the rate of high confidence for differentiating between neoplastic and non-neoplastic colorectal lesions. In other words, magnifying endoscopy with NBI could enhance the application of real-time optical diagnosis in routine clinical practice.
In future, NBI will become a vital tool for detection, characterization and optical biopsy of colorectal polyps, especially when used with magnifying endoscopy.
REFERENCES
1. New diagnostic method based on color imaging using narrow band imaging (NBI) system for gastrointestinal tract. Gastrointest Endosc. 2001; 53:AB125.
2. Masaki T, Katada C, Nakayama M, et al. Narrow band imaging in the diagnosis of intra-epithelial and invasive laryngeal squamous cell carcinoma: a preliminary report of two cases. Auris Nasus Larynx. 2009; 36:712–716.

3. Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004; 9:568–577.

5. Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989; 339:58–61.
6. Aotake T, Lu CD, Chiba Y, Muraoka R, Tanigawa N. Changes of angiogenesis and tumor cell apoptosis during colorectal carcinogenesis. Clin Cancer Res. 1999; 5:135–142.
7. Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996; 44:8–14.

8. Pasha SF, Leighton JA, Das A, et al. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol. 2012; 107:363–370.

9. Nagorni A, Bjelakovic G, Petrovic B. Narrow band imaging versus conventional white light colonoscopy for the detection of colorectal polyps. Cochrane Database Syst Rev. 2012; 1:CD008361.

10. Horimatsu T, Sano Y, Tanaka S, et al. Next-generation narrow band imaging system for colonic polyp detection: a prospective multicenter randomized trial. Int J Colorectal Dis. 2015; 30:947–954.

11. Leung WK, Lo OS, Liu KS, et al. Detection of colorectal adenoma by narrow band imaging (HQ190) vs. high-definition white light colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2014; 109:855–863.

12. Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010; 28:1566–1572.

13. Fu KI, Sano Y, Kato S, et al. Chromoendoscopy using indigo carmine dye spraying with magnifying observation is the most reliable method for differential diagnosis between non-neoplastic and neoplastic colorectal lesions: a prospective study. Endoscopy. 2004; 36:1089–1093.

14. Sano Y, Emura F, Ikematsu H. Narrow band imaging. In : Waye JD, Rex DX, Williams CB, editors. Colonoscopy: Principles and Practice. Oxford: Blackwell;2009. p. 514–526.
15. Sano Y, Ikematsu H, Fu KI, et al. Meshed capillary vessels by use of narrow-band imaging for differential diagnosis of small colorectal polyps. Gastrointest Endosc. 2009; 69:278–283.

16. Katagiri A, Fu KI, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther. 2008; 27:1269–1274.

17. Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol. 2010; 10:33.

18. Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol. 2008; 103:2700–2706.

19. Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009; 136:1174–1181.

20. Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol. 2009; 10:1171–1178.

21. Hewett DG, Kaltenbach T, Sano Y, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012; 143:599–607.e1.

22. Hayashi N, Tanaka S, Hewett DG, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc. 2013; 78:625–632.

23. Iwatate M, Ikumoto T, Hattori S, Sano W, Sano Y, Fujimori T. NBI and NBI combined with magnifying colonoscopy. Diagn Ther Endosc. 2012; 2012:173269.

24. Higashi R, Uraoka T, Kato J, et al. Diagnostic accuracy of narrow-band imaging and pit pattern analysis significantly improved for less-experienced endoscopists after an expanded training program. Gastrointest Endosc. 2010; 72:127–135.

25. Raghavendra M, Hewett DG, Rex DK. Differentiating adenomas from hyperplastic colorectal polyps: narrow-band imaging can be learned in 20 minutes. Gastrointest Endosc. 2010; 72:572–576.

26. Ignjatovic A, Thomas-Gibson S, East JE, et al. Development and validation of a training module on the use of narrow-band imaging in differentiation of small adenomas from hyperplastic colorectal polyps. Gastrointest Endosc. 2011; 73:128–133.

27. Rastogi A, Rao DS, Gupta N, et al. Impact of a computer-based teaching module on characterization of diminutive colon polyps by using narrow-band imaging by non-experts in academic and community practice: a video-based study. Gastrointest Endosc. 2014; 79:390–398.
28. Ladabaum U, Fioritto A, Mitani A, et al. Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology. 2013; 144:81–91.

29. Kuiper T, Marsman WA, Jansen JM, et al. Accuracy for optical diagnosis of small colorectal polyps in nonacademic settings. Clin Gastroenterol Hepatol. 2012; 10:1016–1020.

30. Dai J, Shen YF, Sano Y, et al. Evaluation of narrow-band imaging in the diagnosis of colorectal lesions: is a learning curve involved? Dig Endosc. 2013; 25:180–188.

31. Machida H, Sano Y, Hamamoto Y, et al. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy. 2004; 36:1094–1098.

32. Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ, Chiu CT. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol. 2006; 101:2711–2716.

33. Apel D, Jakobs R, Schilling D, et al. Accuracy of high-resolution chromoendoscopy in prediction of histologic findings in diminutive lesions of the rectosigmoid. Gastrointest Endosc. 2006; 63:824–828.

34. Tischendorf JJ, Wasmuth HE, Koch A, Hecker H, Trautwein C, Winograd R. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy. 2007; 39:1092–1096.

35. De Palma GD, Rega M, Masone S, et al. Conventional colonoscopy and magnified chromoendoscopy for the endoscopic histological prediction of diminutive colorectal polyps: a single operator study. World J Gastroenterol. 2006; 12:2402–2405.
36. Rex DK, Kahi C, O’Brien M, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011; 73:419–422.

37. ASGE Technology Committee, Abu Dayyeh BK, Thosani N, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2015; 81:502.e1–502.e16.

Fig. 1.
Comparison of the field of view between the Olympus 260 and 290 series colonoscopes. The newly developed 290 series colonoscopes have a 170º field of view, 30º wider than the previous 260 series colonoscopes. This can help physicians to detect mucosal changes more rapidly, with less need for angulation.
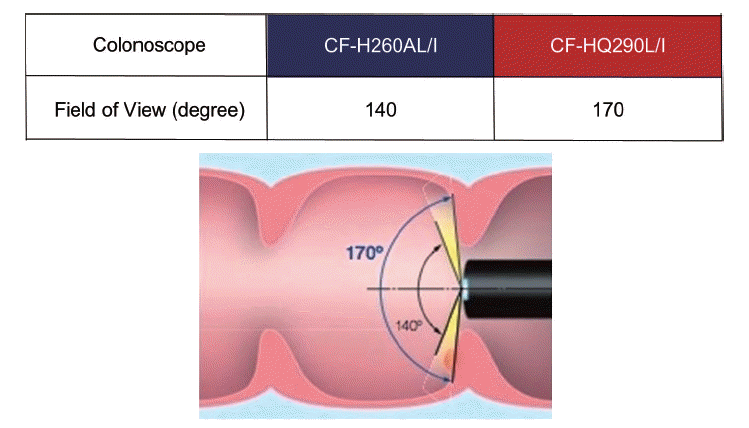
Fig. 2.
Comparison of colonoscopic views using narrow-band imaging (NBI) between the Olympus 260 and 290 series endoscopy systems. (A) Colonoscopic view using NBI in the Olympus 260 series endoscopy system (EVIS LUCERA SPECTRUM). (B) Colonoscopic view using NBI in the Olympus 290 series endoscopy system (EVIS LUCERA ELITE). Colonoscopic views using NBI in the newly developed 290 series endoscopy system are brighter and have a higher resolution than those in the 260 series.
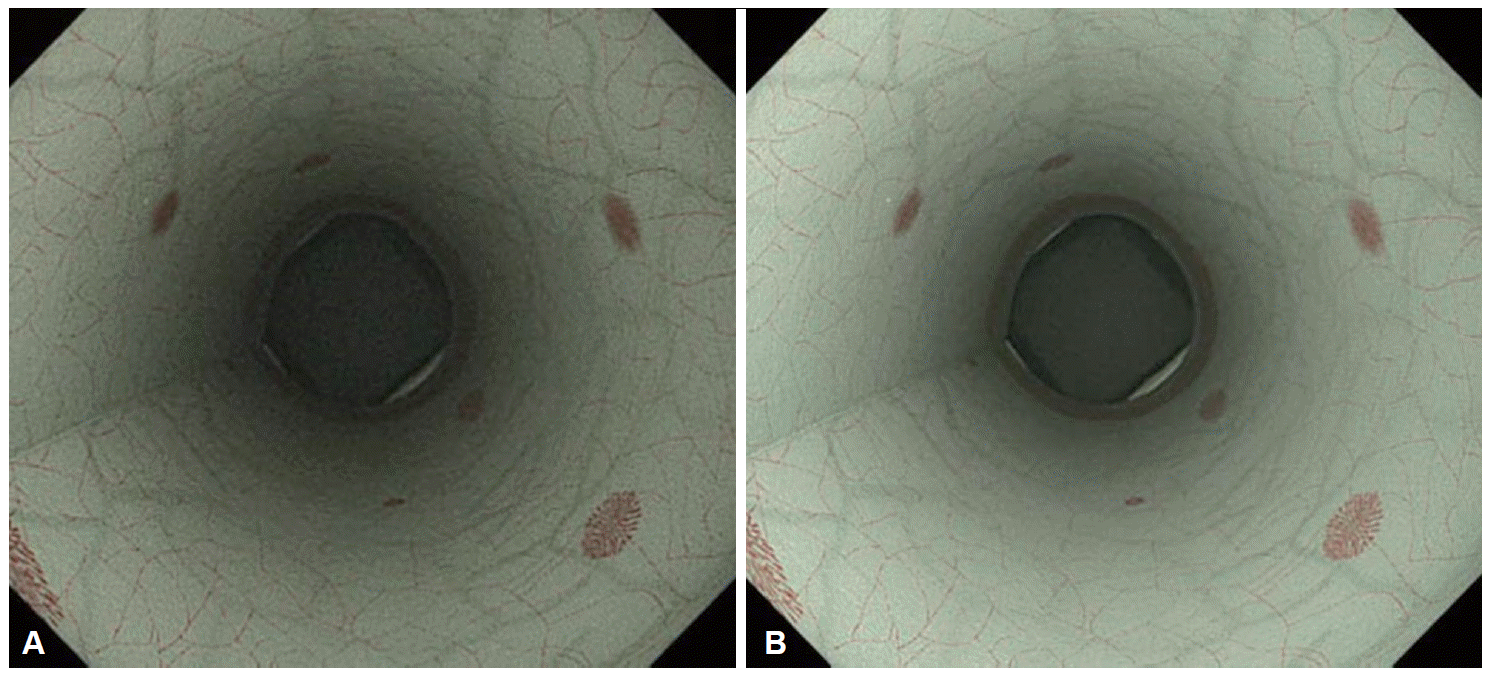
Fig. 3.
Colonoscopic views using high-definition white light (HD-WL) and high-definition narrow-band imaging (HD-NBI) in the Olympus 290 series endoscopy system. (A) Colonoscopic view using HD-WL. (B) Colonoscopic view using HD-NBI. Colorectal neoplastic lesions are recognized as pale-reddish or reddish areas using WL, and as brownish areas using NBI, which is caused by mucosal microvascular dilatation and neoplastic angiogenesis. White arrows indicate neoplastic lesions.
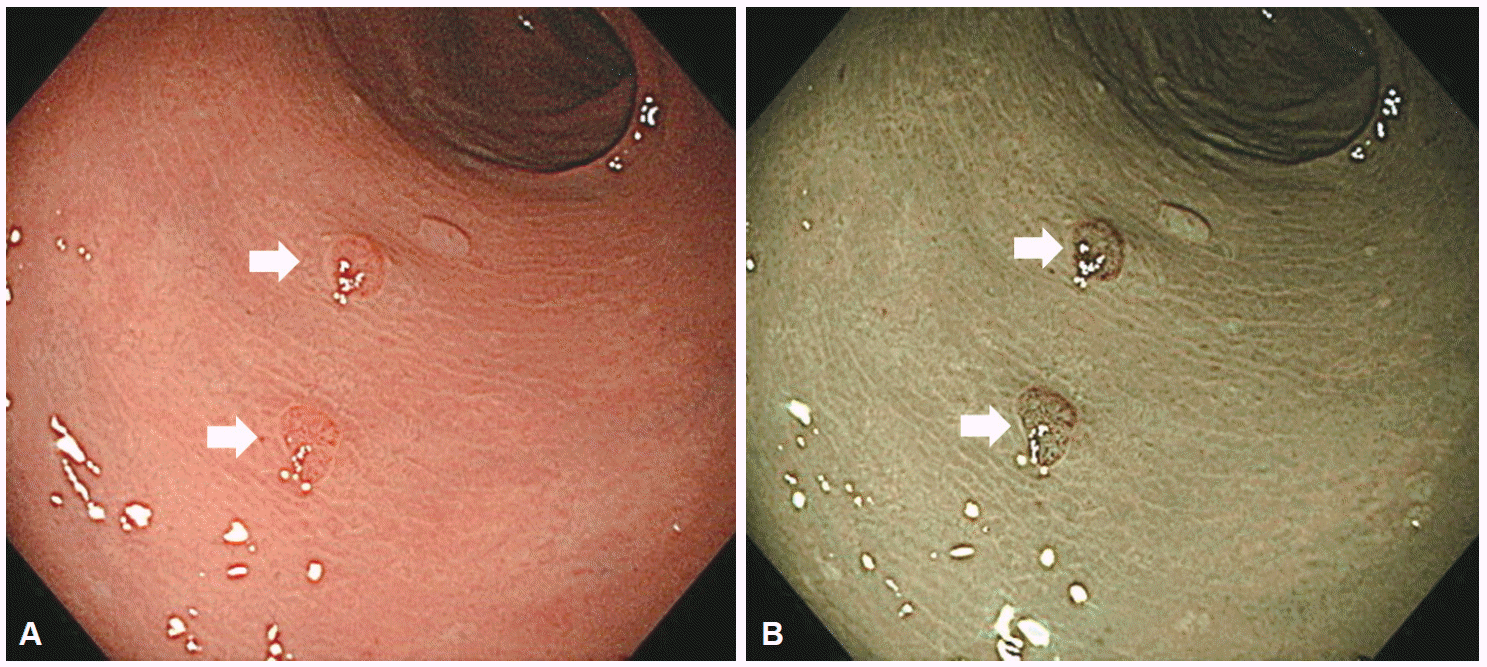
Fig. 5.
Three-step strategy of narrow-band imaging (NBI). Adapted from Iwatate et al. [23] ELITE, Olympus 290 series endoscopy system EVIS LUCERA ELITE; NICE, NBI International Colorectal Endoscopic.
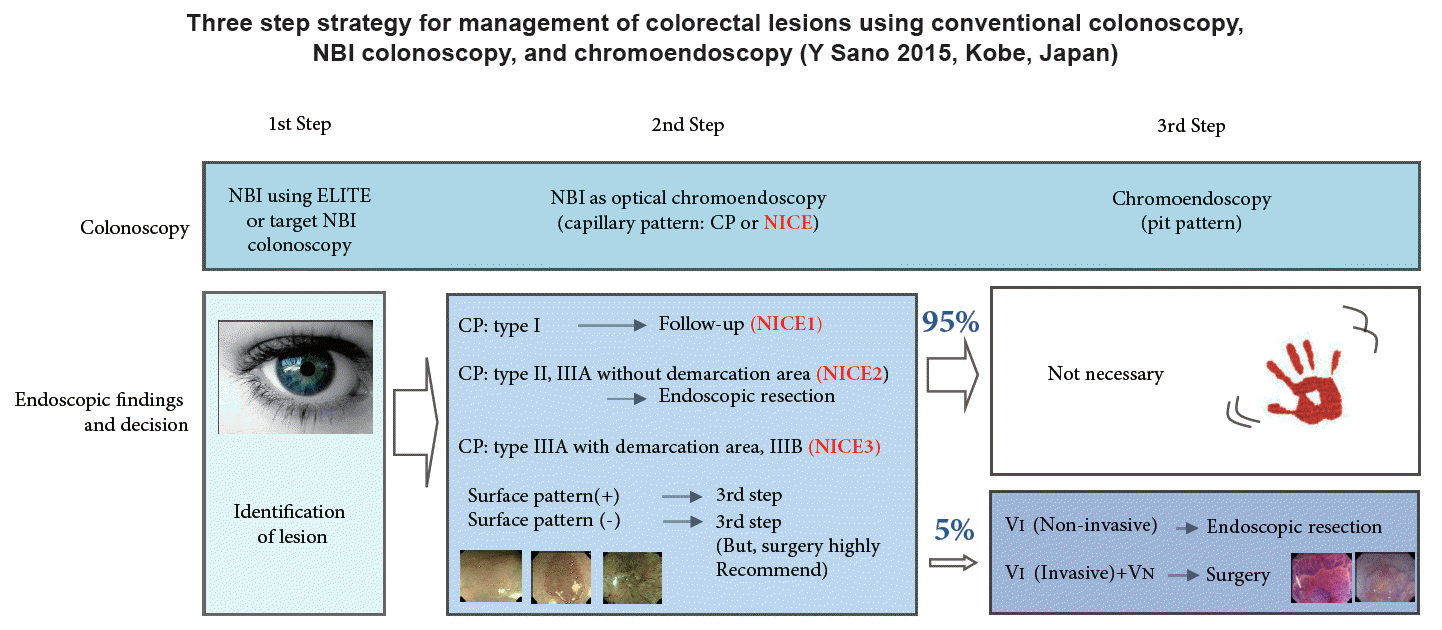
Table 1.
NICE classification
| Type 1 | Type 2 | Type 3 | |
|---|---|---|---|
| Color | Same or lighter than background | Browner relative to background (verify color arises from vessels) | Brown to dark brown relative to background; sometimes patchy whiter areas |
| Vessels | None, or isolated lacy vessels may be present coursing across the lesion | Brown vessels surrounding white structuresa) | Has area(s) of disrupted or missing vessels |
| Surface pattern | Dark or white spots of uniform size, or homogeneous absence of pattern | Oval, tubular or branched white structuresb) surrounded by brown vessels | Amorphous or absent surface pattern |
| Most likely pathology | Hyperplastic & sessile serrated polypc) | Adenomad) | Deep submucosal invasive cancer |
b) These structures (regular or irregular) may represent the pits and the epithelium of the crypt opening;
c) In the World Health Organization classification, sessile serrated polyp and sessile serrated adenoma are synonymous;
d) Type 2 consists of Vienna classification types 3, 4, and superficial 5 (all adenomas with either low or high grade dysplasia, or with superficial submucosal carcinoma). The presence of high grade dysplasia or superficial submucosal carcinoma may be suggested by an irregular vessel or surface pattern, and is often associated with atypical morphology (e.g., depressed area).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download