Abstract
Magnifying endoscopy with narrow-band imaging (M-NBI) can visualize superficial microanatomies in the stomach. The normal morphology of the microanatomy visualized by M-NBI differs according to the part of the stomach. The gastric fundic glandular mucosa appears as a regular honeycomb-like subepithelial capillary network (SECN) pattern with a regular collecting venule pattern and regular oval crypt opening with circular marginal crypt epithelium (MCE) pattern. The gastric pyloric glandular mucosa displays a regular coil-shaped SECN pattern and regular polygonal or curved MCE pattern. For a diagnosis of early gastric cancer using M-NBI, the vessel plus surface classification system was developed. This system is clinically useful for the differential diagnosis of focal gastritis and small depressed cancer and for determining the horizontal extent of early gastric cancer for successful endoscopic resection. Advantages of M-NBI over conventional endoscopic imaging techniques with white light include accurate diagnosis and cost effectiveness. This technique is a breakthrough in the endoscopic diagnostic field.
Magnifying endoscopy is a powerful modality since it can visualize real-time microscopic images of the mucosal surface. The most advanced magnifying endoscopy has a maximal resolution power as small as 6.4 micrometers [1]; thus, allowing the dissection of a capillary, the smallest unit of blood vessels in the human body. The era has changed from pattern analysis to anatomical investigation using magnifying endoscopy. In addition, narrow-band imaging (NBI) had been developed and incorporated into the magnifying endoscopy system. The basic principles have been described by Dr. K Gono in this issue of Clinical Endoscopy. When we focus on clinical impact, we are confident that we can obtain clear images of both the microvascular architecture (V) and the microsurface structure (S). In this article, I would like to introduce clinical usefulness of magnifying narrow-band imaging (M-NBI) based on outcomes from systematic clinical trials.
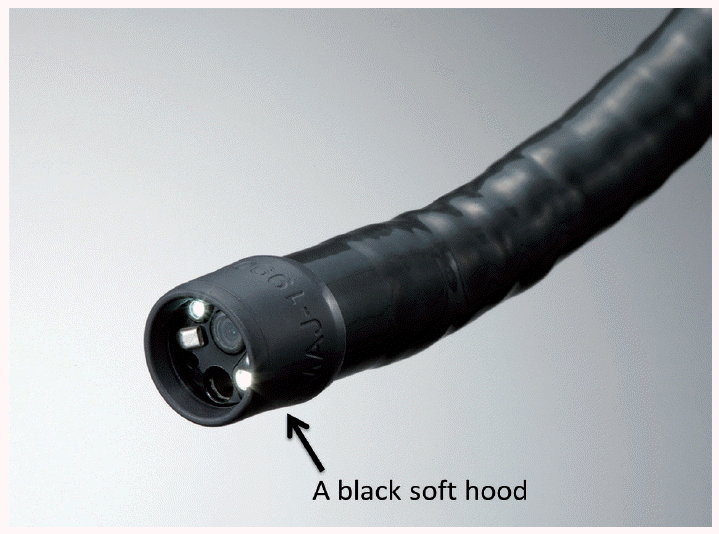
A soft black hood (MAJ-1988 for GIF-Q240Z and GIF-H290Z; MAJ-1989 or GIF-H260Z and GIF-Q160Z; Olympus, Tokyo, Japan) is necessary for optimal magnifying endoscopic examinations [1]. Prior to the procedure, the hood is mounted onto the tip of the endoscope to enable the endoscopist to consistently fix the mucosa at a distance of approximately 2 mm, at which maximal magnification of the endoscopic image can be obtained (Fig. 1). Since the depth of the hood is very shallow, it does not disturb the visual field during non-magnifying observations. Without a hood, it is impossible to quickly and easily obtain properly focused images in the stomach with its broad lumen, as well as eventual interference from respiratory movements and aortic pulsations. In other words, using a hood, we can easily obtain magnified images of the required resolution. The hood is soft enough not to injure the mucosa. Indeed, when upper gastrointestinal magnification endoscopy was performed in more than 600 screening endoscopy cases using this type of soft black hood, there were no complications such as contact bleeding, nor any untoward incidents such as hood dislocation [2].
Each microanatomical component in the stomach visualized by M-NBI is common; however, its morphology varies depending upon the part of the stomach visualized, that is, whether gastric fundic glandular mucosa (Fig. 2) or gastric pyloric glandular mucosa (Fig. 3) is being examined [3]. The important concept is that the analysis of magnified endoscopic findings should be made based on a description using anatomical terms. With respect to the V, the subepithelial capillary network (SECN), the collecting venule (CV), and the microvessel (MV) are visualized [4]. The term microvessels should be applied when the vessels cannot be categorized as either capillary or venule in a pathological condition. For the S, the marginal crypt epithelium (MCE), the crypt opening (CO), and the intervening part between crypts are the major anatomical components [4].
As for V, the morphology of the capillary is that of a dark brown polygonal closed loop (Fig. 2). These loops anastomose repeatedly with each other, forming a regular honeycomb-like SECN pattern. The SECN drains into a cyan-colored CV [1,4,5]. In the normal fundic glandular mucosa without Helicobacter pylori infection, the regular CV is consistently preserved [6,7]. As for S, the epithelial morphology is visualized as semitransparent white belt-like structures, showing a circular or oval shape. This is the MCE. In the center, the brown oval CO is surrounded by the MCE [1,4,5].
As for V, the morphology of the capillary is that of a dark brown coil-shaped open loop (Fig. 3) [1,4,5]. Although it has been shown anatomically that these loops anastomose repeatedly with each other under the epithelial surface, subepithelial anastomoses are not often visualized [1,4,5]. In contrast to the gastric fundic glandular mucosa, CVs are rarely observed since they are located at the deeper part of the mucosa [1,4,5].
In some pathological conditions, the presence of a light blue crest (Fig. 4A) or a white opaque substance (WOS) (Fig. 4B) can be markers for S. These two findings are very useful objective markers for the presence of intestinal metaplasia [8-10]. The WOS can be an alternative marker for making a diagnosis of early gastric cancer (EGC) when the subepithelial microvascular (MV) pattern is obscured by the WOS [9].
The vessel plus surface (VS) classification system is based on the ability of M-NBI to clearly visualize both MV and microsurface (MS) patterns [1]. This diagnostic system has proven to be very useful for the correct diagnosis of superficial (0-II) cancer [2,11-21] and the delineation of the margins of EGC [12,22,23]. This VS classification system is a well-accepted systematic method worldwide for the diagnosis of EGC using magnifying endoscopy.
There are three categories of MV and MS patterns including regular, irregular, and absent (Fig. 5) [1,24]. In the regular MV pattern, mucosal capillaries have a uniform shape that can be closed-looped (polygonal) or open-looped with a homogeneous morphology, symmetrical distribution, and regular arrangement. In the irregular MV pattern, the vessels are closed-looped (polygonal), open-looped, tortuous, branched, or bizarrely shaped, with or without a network, and have a heterogeneous morphology, asymmetrical distribution, and irregular arrangement. In the absent MV pattern, the subepithelial MV pattern is obscured by a WOS within the superficial mucosa. A regular MS pattern is defined when the MCE is of a uniform linear/curved/oval/circular structure with a homogeneous morphology, symmetrical distribution, and regular arrangement. When the MCE is an irregular linear/curved/oval/circular/villous structure with a heterogeneous morphology, asymmetrical distribution, and irregular arrangement, it is defined as an irregular MS pattern. In the absent MS pattern, neither the marginal crypt epithelial structure nor the WOS is visible using M-NBI.
According to the VS classification system, the characteristic M-NBI findings of EGC are a clear demarcation line between non-cancerous and cancerous mucosa, and an irregular MV pattern and/or irregular MS pattern within the demarcation line [1,24]. Accordingly, we set two criteria for diagnosing EGC: (1) an irregular MV pattern with a demarcation line and/or (2) an irregular MS pattern with a demarcation line [1,24]. If either or both criteria are fulfilled, an endoscopic diagnosis can be made. According to our investigations, 97% of EGC fit these criteria [1].
In practice, when we detect a suspicious lesion, we should first determine whether a demarcation line is present. If the demarcation line is absent, it is not likely a cancerous lesion since the presence of a demarcation line has high sensitivity (95%) for predicting the cancerous lesion [19]. When a demarcation line is identified we need to determine the VS classification focusing on the MV and MS patterns within the demarcation line, since the presence of a demarcation line alone does not show enough specificity (49%) for the diagnosis of a cancerous lesion [19]. If an irregular MV pattern and/or an irregular MS pattern can be identified, the lesion is diagnosed as a cancerous lesion since these findings demonstrate quite high specificity (95%) [19,20]. If either an irregular MV pattern or an irregular MS pattern is absent, the lesion is diagnosed as non-cancerous [1,19,20,24]. The principle of the VS classification is that we should analyze MV and MS patterns separately and then determine whether or not, the obtained magnified endoscopic findings meet the criteria described above [1,24].
Making a differential diagnosis of slightly depressed lesion between focal gastritis and small depressed cancer used to be one of the limitations of conventional white light imaging (C-WLI). We investigated the real-time diagnostic yield of the C-WLI compared to the M-NBI in a multicenter, prospective, randomized controlled trial of patient with undiagnosed depressed lesions ≤10 mm in diameter detected during screening esophagogastroduodenoscopy [14]. With regards to the diagnostic performance of C-WLI versus M-NBI for small, depressed gastric lesions, the accuracy and specificity of M-NBI were greater than with C-WLI, although the difference in sensitivity was not significant (Table 1). The combination of C-WLI and M-NBI significantly enhanced performance compared with the C-WLI alone (Table 2). These outcomes clearly demonstrate that carrying out M-NBI after careful C-WLI has sufficient diagnostic accuracy for determining a correct endoscopic diagnosis even for gastritis-like cancer (Fig. 6). Furthermore, we demonstrated that the M-NBI has greater sensitivity and reproducibility than chromoendoscopy (CE) for the diagnosis of minute (≤5 mm diameter) gastric cancers [21].
Nevertheless, in clinical practice, taking a biopsy cannot be omitted, because the histological findings, such as histological type, are needed for a diagnosis of cancer. However, using the M-NBI and the VS classification system could contribute towards minimization of the number of biopsies of noncancerous lesions taken in order to rule out gastritis-like cancer. In fact, we have demonstrated the advantages in cost-effectiveness of M-NBI, in particular, M-NBI may contribute to reducing the number of biopsies required to detect a cancer in screening endoscopy [20].
We also investigated the limitations of M-NBI in screening endoscopy by a prospective multicenter feasibility study. As shown in Fig. 7, a pale depressed lesion by C-WLI is characteristic of a signet-ring cell or undifferentiated carcinoma [20]. By M-NBI, it is only visualized as a regular MV pattern and a regular MS pattern with a demarcation line. Since a signet-ring cell carcinoma invades and spreads sparsely underneath the surface epithelium without any destruction of the mucosal epithelium and subepithelial capillary, no specific change is observed in magnified endoscopic findings [20]. Considering that a flat or depressed pale lesion is not an indication of M-NBI in screening endoscopy, on the contrary a good indication may be the presence of a non-pale lesion. In such conditions, the M-NBI can be considered an optical biopsy [20].
Magnifying endoscopy enables reliable delineation of the horizontal extent of EGCs prior to endoscopic submucosal dissection (ESD) [22,23]. In our previous studies, we have reported the advantages of M-NBI over C-WLI with dye spraying CE [23]. We investigated the usefulness and limitations of ME with NBI when CE could not be used to determine the horizontal extent of an EGC. A series of 350 consecutive EGCs resected en bloc using ESD were included in the study. Approximately 18.9% (66/350) of cases showed unclear margins using CE. Of these, 62 of 66 cancers were examined using M-NBI. According to the VS classification system, the entire margins were successfully delineated in 72.6% (45/62) of the lesions with unclear margins using CE (Fig. 8) [23]. However, the diagnostic success rate for undifferentiated cancers was 0%, significantly lower than that for differentiated lesions (p<0.001). Accordingly, M-NBI using the VS classification system is an excellent modality for identifying the entire margin of EGCs when the margins are unclear using CE. However, it is still difficult to assess the lateral extent of the EGC of an undifferentiated type using endoscopic findings alone because the cancer cell infiltrates laterally in the lamina propria deep to the glandular neck. M-NBI of the surface cannot detect any cancer with specific irregular MV or MS patterns. Thus, the endoscopic diagnostic strategy to be adopted depends on the histological type, as shown in Fig. 9. In difficult cases, we recommend that the clinical strategy should be to take biopsy samples from the apparently noncancerous tissue around the lesion and then determine the resection margins after histopathologically confirming the absence of cancerous invasion in the biopsy specimens.
Since M-NBI became available in clinical practice in 2006, there have been numerous reports describing the clinical usefulness or investigations. Since the extent of this literature is limited. Below, I will list the major clinical applications in addition to those described above along with references to the representative papers.
(3) Diagnosis of papillary adenocarcinoma, using the marker VEC (vessel within an epithelial circle) pattern [27]
M-NBI is a promising endoscopic technique since it is safe, quick, and accurate for making a correct diagnosis of a small or flat gastric lesions which used be difficult by conventional endoscopy alone. The VS classification system for the diagnosis of EGC is very useful in clinical practice. Clinical applications of M-NBI are well established for the differential diagnosis of focal gastritis and small depressed cancer and for determining the horizontal extent of EGC for successful application of ESD. Other suggested clinical applications need to be tested for reproducibility and importance of findings by well-designed clinical trials.
REFERENCES
1. Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009; 41:462–467.

2. Yao K, Iwashita A, Tanabe H, et al. Novel zoom endoscopy technique for diagnosis of small flat gastric cancer: a prospective, blind study. Clin Gastroenterol Hepatol. 2007; 5:869–878.

3. Yao K, Oishi T. Microgastroscopic findings of mucosal microvascular architecture as visualized by magnifying endoscopy. Dig Endosc. 2001; 13(Suppl 1):S27–S33.

4. Yao K. Microanatomies as visualized using magnifying endoscopy with narrow band imaging in the stomach: which microanatomical structures can we visualize in the glandular epithelium using narrow band imaging, and how is this achieved?. In : Yao K, editor. Zoom Gastroscopy. Tokyo: Springer;2013. p. 57–69.
5. Yao K. Clinical applications of magnifying endoscopy with narrow-band imaging (M-NBI) of the stomach. In : Yao K, editor. Zoom Gastroscopy. Tokyo: Springer;2013. p. 83–87.
6. Nakagawa S, Kato M, Shimizu Y, et al. Relationship between histopathologic gastritis and mucosal microvascularity: observations with magnifying endoscopy. Gastrointest Endosc. 2003; 58:71–75.

7. Anagnostopoulos GK, Yao K, Kaye P, et al. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007; 39:202–207.

8. Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006; 38:819–824.

9. Yao K, Iwashita A, Tanabe H, et al. White opaque substance within superficial elevated gastric neoplasia as visualized by magnification endoscopy with narrow-band imaging: a new optical sign for differentiating between adenoma and carcinoma. Gastrointest Endosc. 2008; 68:574–580.

10. Matsushita M, Mori S, Uchida K, Nishio A, Okazaki K. “White opaque substance” and “light blue crest” within gastric flat tumors or intestinal metaplasia: same or different signs? Gastrointest Endosc. 2009; 70:402.

11. Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc. 2002; 56:279–284.

12. Yao K, Iwashita A, Kikuchi Y, et al. Novel zoom endoscopy technique for visualizing the microvascular architecture in gastric mucosa. Clin Gastroenterol Hepatol. 2005; 3(7 Suppl 1):S23–S26.

13. Ezoe Y, Muto M, Horimatsu T, et al. Magnifying narrow-band imaging versus magnifying white-light imaging for the differential diagnosis of gastric small depressive lesions: a prospective study. Gastrointest Endosc. 2010; 71:477–484.

14. Ezoe Y, Muto M, Uedo N, et al. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology. 2011; 141:2017–2025.

15. Miwa K, Doyama H, Ito R, et al. Can magnifying endoscopy with narrow band imaging be useful for low grade adenomas in preoperative biopsy specimens? Gastric Cancer. 2012; 15:170–178.

16. Maki S, Yao K, Nagahama T, et al. Magnifying endoscopy with narrow-band imaging is useful in the differential diagnosis between lowgrade adenoma and early cancer of superficial elevated gastric lesions. Gastric Cancer. 2013; 16:140–146.

17. Yao K. How is the VS (vessel plus surface) classification system applicable to magnifying narrow-band imaging examinations of gastric neoplasias initially diagnosed as low-grade adenomas? Gastric Cancer. 2012; 15:118–120.

18. Tao G, Xing-Hua L, Ai-Ming Y, et al. Enhanced magnifying endoscopy for differential diagnosis of superficial gastric lesions identified with white-light endoscopy. Gastric Cancer. 2014; 17:122–129.

19. Yamada S, Doyama H, Yao K, et al. An efficient diagnostic strategy for small, depressed early gastric cancer with magnifying narrow-band imaging: a post-hoc analysis of a prospective randomized controlled trial. Gastrointest Endosc. 2014; 79:55–63.
20. Yao K, Doyama H, Gotoda T, et al. Diagnostic performance and limitations of magnifying narrow-band imaging in screening endoscopy of early gastric cancer: a prospective multicenter feasibility study. Gastric Cancer. 2014; 17:669–679.

21. Fujiwara S, Yao K, Nagahama T, et al. Can we accurately diagnose minute gastric cancers (</=5 mm)? Chromoendoscopy (CE) vs magnifying endoscopy with narrow band imaging (M-NBI). Gastric Cancer. 2015; 18:590–596.
22. Yao K, Yao T, Iwashita A. Determining the horizontal extent of early gastric carcinoma: two modern techniques based on differences in the mucosal microvascular architecture and density between carcinomatous and non-carcinomatous mucosa. Dig Endosc. 2002; 14(Suppl 1):S83–S87.

23. Nagahama T, Yao K, Maki S, et al. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Gastrointest Endosc. 2011; 74:1259–1267.

24. Yao K. The vessels plus surface (VS) classification system in the diagnosis of early gastric cancer. In : Yao K, editor. Zoom Gastroscopy. Tokyo: Springer;2013. p. 89–98.
25. Kanesaka T, Sekikawa A, Tsumura T, et al. Dense-type crypt opening seen on magnifying endoscopy with narrow-band imaging is a feature of gastric adenoma. Dig Endosc. 2014; 26:57–62.

26. Kanzaki H, Uedo N, Ishihara R, et al. Comprehensive investigation of areae gastricae pattern in gastric corpus using magnifying narrow band imaging endoscopy in patients with chronic atrophic fundic gastritis. Helicobacter. 2012; 17:224–231.

27. Kanemitsu T, Yao K, Nagahama T, et al. The vessels within epithelial circle (VEC) pattern as visualized by magnifying endoscopy with narrow-band imaging (ME-NBI) is a useful marker for the diagnosis of papillary adenocarcinoma: a case-controlled study. Gastric Cancer. 2014; 17:469–477.

28. Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004; 36:1080–1084.

29. Kanesaka T, Sekikawa A, Tsumura T, et al. Absent microsurface pattern is characteristic of early gastric cancer of undifferentiated type: magnifying endoscopy with narrow-band imaging. Gastrointest Endosc. 2014; 80:1194–1198.

30. Doyama H, Yoshida N, Tsuyama S, et al. The “white globe appearance” (WGA): a novel marker for a correct diagnosis of early gastric cancer by magnifying endoscopy with narrow-band imaging (M-NBI). Endosc Int Open. 2015; 3:E120–E124.

31. Yoshida N, Doyama H, Nakanishi H, et al. White globe appearance is a novel specific endoscopic marker for gastric cancer: a prospective study. Dig Endosc. 2015 Jul 31 [Epub]. http://dx.doi.org/10.1111/den.12519.

32. Yao K, Iwashita A, Nambu M, et al. Nature of white opaque substance in gastric epithelial neoplasia as visualized by magnifying endoscopy with narrow-band imaging. Dig Endosc. 2012; 24:419–425.

33. Ueo T, Yonemasu H, Yada N, et al. White opaque substance represents an intracytoplasmic accumulation of lipid droplets: immunohistochemical and immunoelectron microscopic investigation of 26 cases. Dig Endosc. 2013; 25:147–155.

34. Ueyama H, Matsumoto K, Nagahara A, et al. A white opaque substance-positive gastric hyperplastic polyp with dysplasia. World J Gastroenterol. 2013; 19:4262–4266.

35. Ohtsu K, Yao K, Matsunaga K, et al. Lipid is absorbed in the stomach by epithelial neoplasms (adenomas and early cancers): a novel functional endoscopy technique. Endosc Int Open. 2015; 3:E318–E322.

36. Enjoji M, Kohjima M, Ohtsu K, et al. Intracellular mechanisms underlying lipid accumulation (white opaque substance) in gastric epithelial neoplasms: a pilot study of expression profiles of lipid-metabolism-associated genes. J Gastroenterol Hepatol. 2015 Oct 29 [Epub]. http://dx.doi.org/10.1111/jgh.13216.

Fig. 2.
(A) Magnifying endoscopy with narrow-band imaging (M-NBI) findings of the normal gastric fundic gland mucosa. V (microvascular architecture): regular honeycomb-like subepithelial capillary network (SECN) pattern with regular collecting venule (CV) pattern is present. S (microsurface structure): regular oval crypt opening (CO) pattern with circular marginal crypt epithelium (MCE) pattern. (B) Anatomical components visualized by M-NBI in the normal gastric fundic gland mucosa. (C) Correlation between visualized microanatomies by M-NBI (upper part) and histological findings (lower part) in the superficial part of the gastric fundic glandular mucosa. SEC, subepithelial capillary; IP, intervening part between crypts.
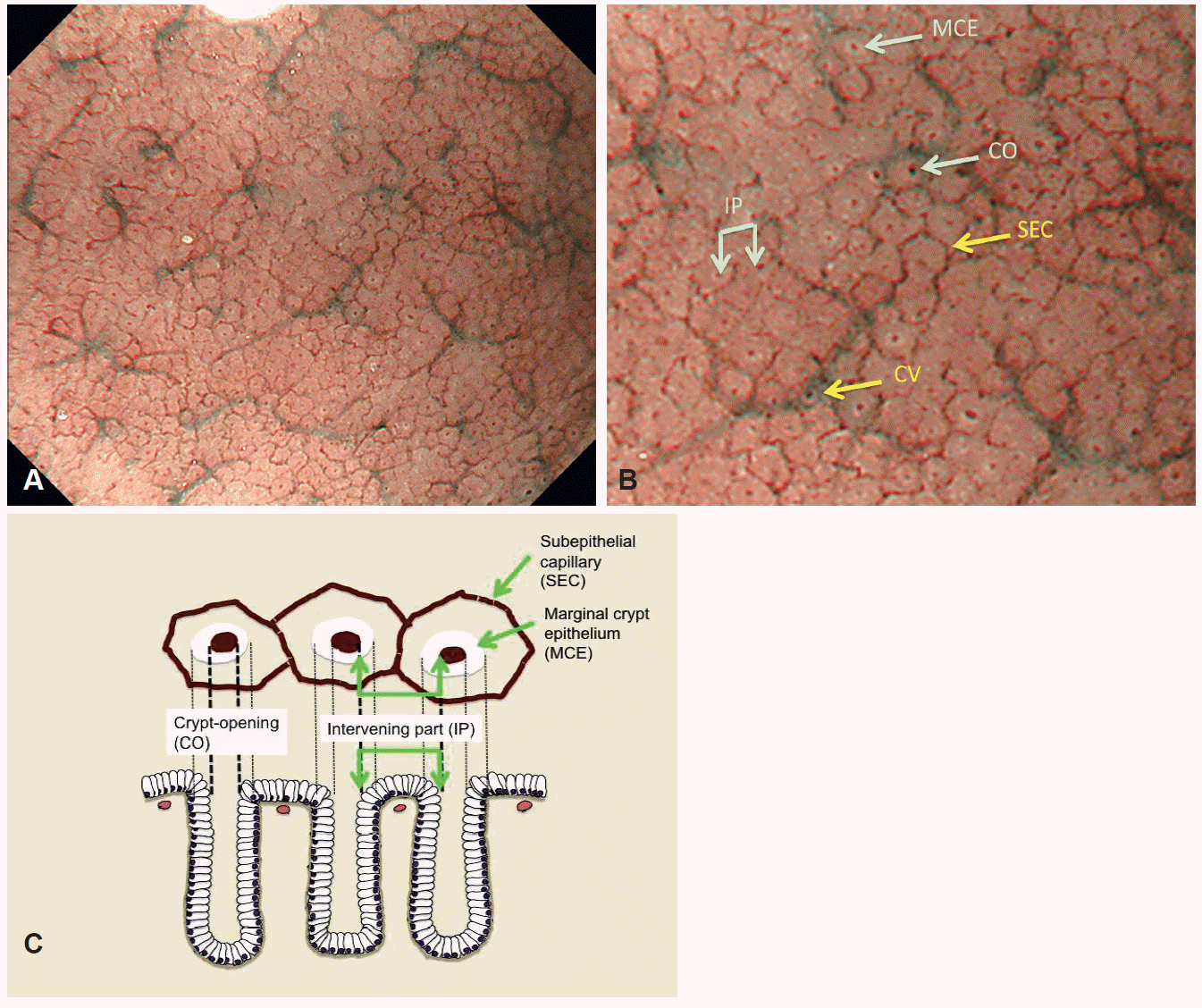
Fig. 3.
(A) Magnifying endoscopy with narrow-band imaging (M-NBI) findings of the normal gastric pyloric gland mucosa. V (microvascular architecture): regular coil-shaped subepithelial capillary (SEC) network pattern but absent regular collecting venule pattern. S (microsurface structure): regular polygonal or curved marginal crypt epithelium (MCE) pattern. (B) Anatomical components visualized by M-NBI in the normal gastric fundic gland mucosa. (C) Correlation between visualized microanatomies by M-NBI (upper part) and histological findings (lower part) in the superficial part of the gastric pyloric glandular mucosa. IP, intervening part between crypts.
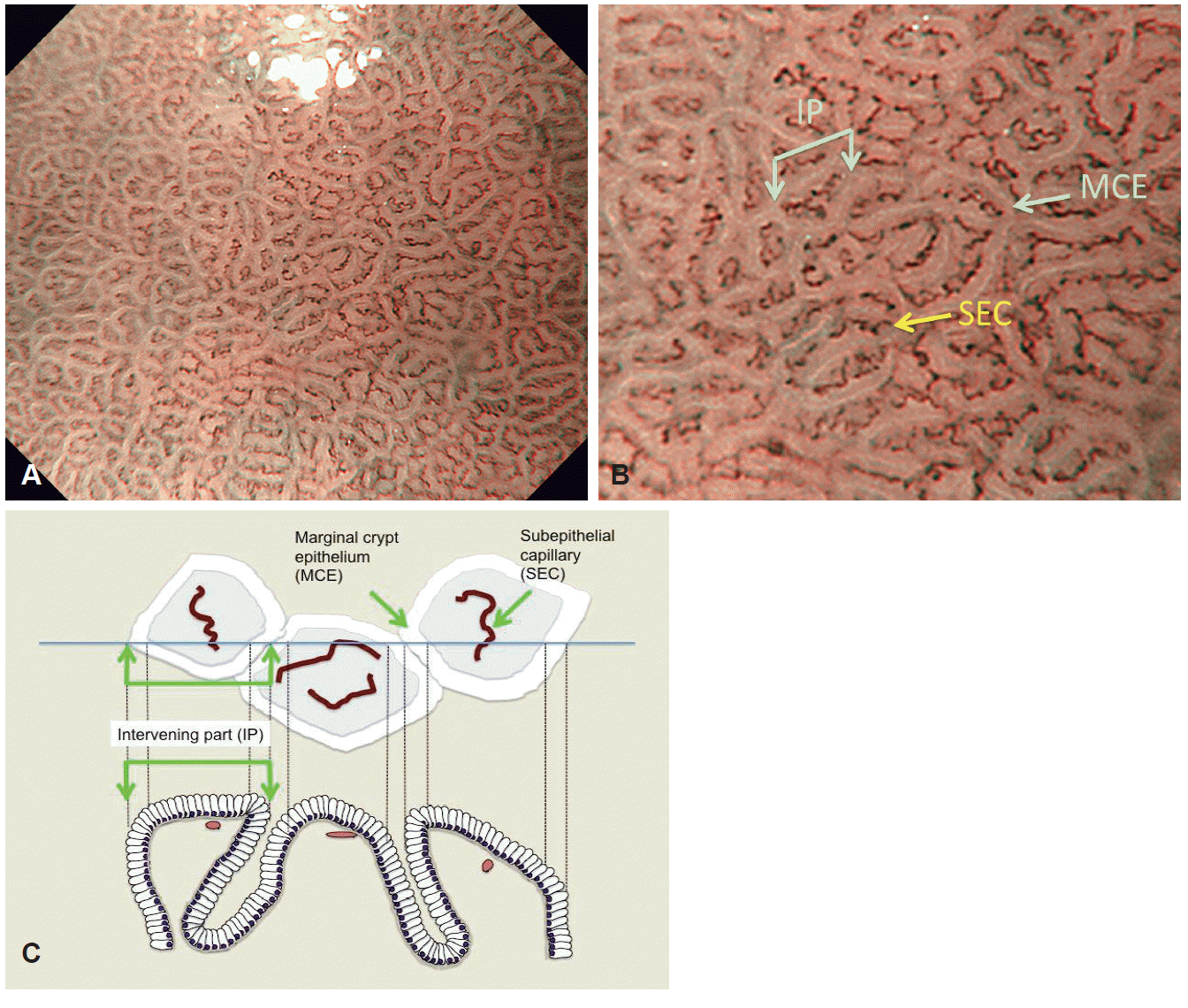
Fig. 4.
(A) Magnifying endoscopy with narrow-band imaging (M-NBI) findings of light blue crest (LBC) in gastric mucosa with intestinal metaplasia. Fine light blue (light cyan colored) linear reflections are located on the epithelial margins, visualized using M-NBI. (B) M-NBI findings of white opaque substance (WOS) in gastric mucosa with intestinal metaplasia. WOS visualized by reflections/strong scattering of whole projected lights located in the surface epithelium of the intervening part.
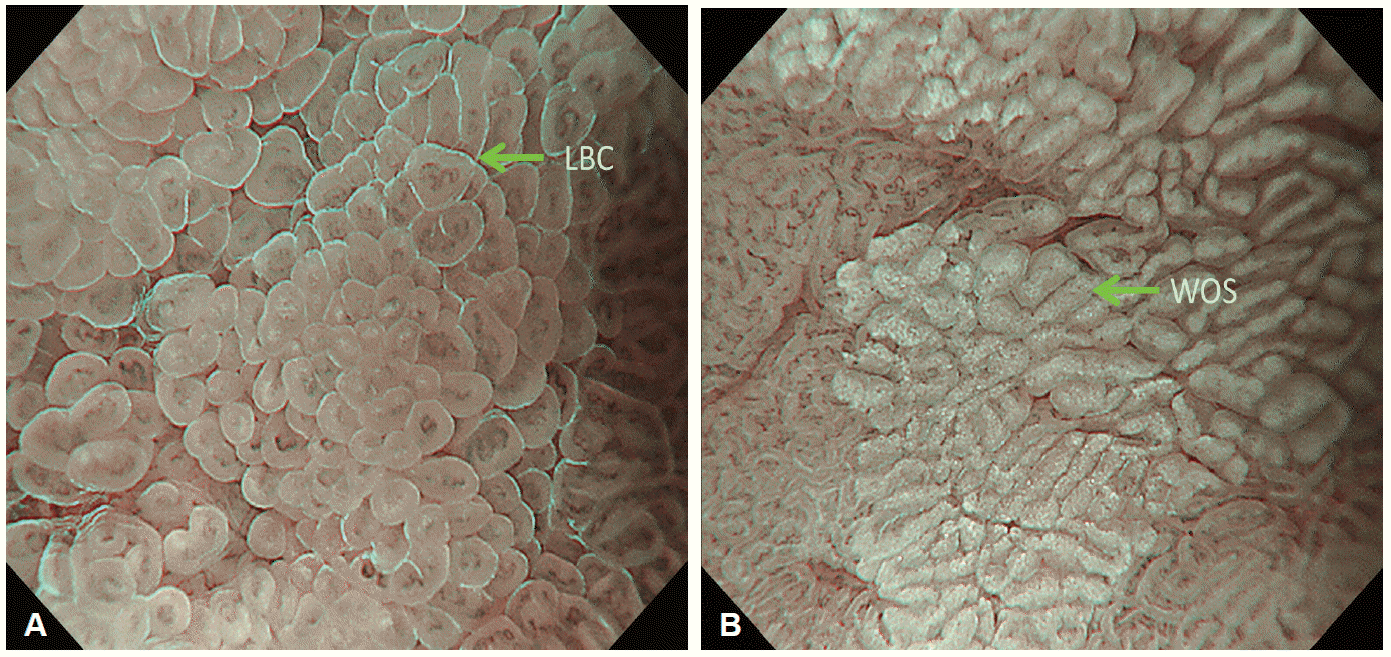
Fig. 5.
Vessel plus surface (VS) classification. Adapted from Yao et al., [1] with permission from Thieme.
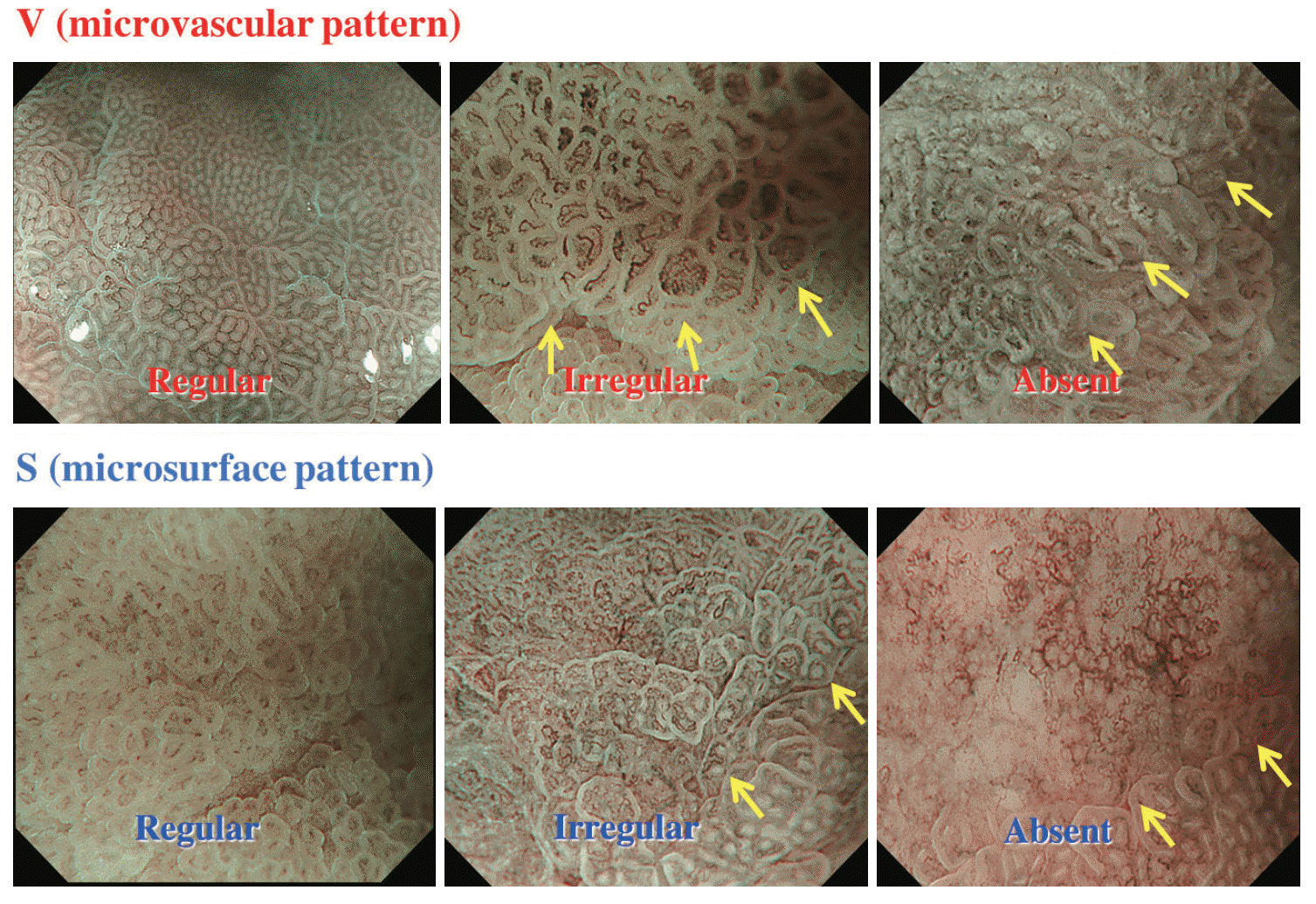
Fig. 6.
Magnifying endoscopy with narrow-band imaging (M-NBI) findings of small depressed gastric lesions. (A) An example of non-cancerous lesion. A distinct demarcation line (arrows) is detected between the background mucosa and the depressed lesion. Within the demarcation line, it shows regular microvascular (MV) pattern plus regular microsurface (MS) pattern according to the vessel plus surface classification. Histological findings of biopsy specimen showed non-cancerous lesion (chronic gastritis with intestinal metaplasia). (B) An example of cancerous lesion. A demarcation line (arrows) is present between the background mucosa and the lesion. Within the demarcation line, irregular MV pattern plus irregular MS pattern is identified. Histological findings of endoscopically resected specimens demonstrated well-differentiated adenocarcinoma (2 mm in diameter). Adapted from Fujiwara et al., [21] with permission from Springer.
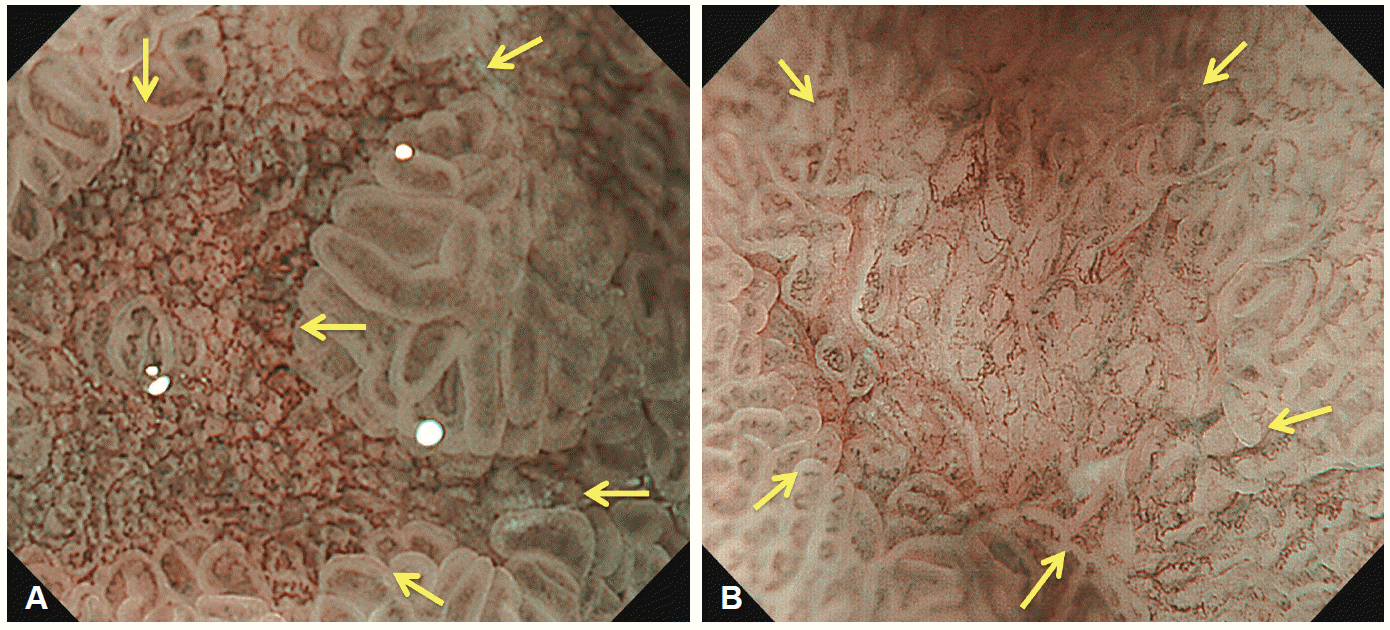
Fig. 7.
A good example of limitations of magnifying endoscopy with narrow-band imaging (M-NBI) in screening endoscopy. (A) Conventional white light imaging shows a slightly depressed pale lesion in the gastric antrum. (B) M-NBI demonstrates regular microvascular pattern plus regular microsurface pattern with a demarcation line. (C) The histological findings of the biopsy specimen show signet-ring cell carcinoma (H&E stain, ×25). Adapted from Nagahama et al., [23] with permission from Elsevier.
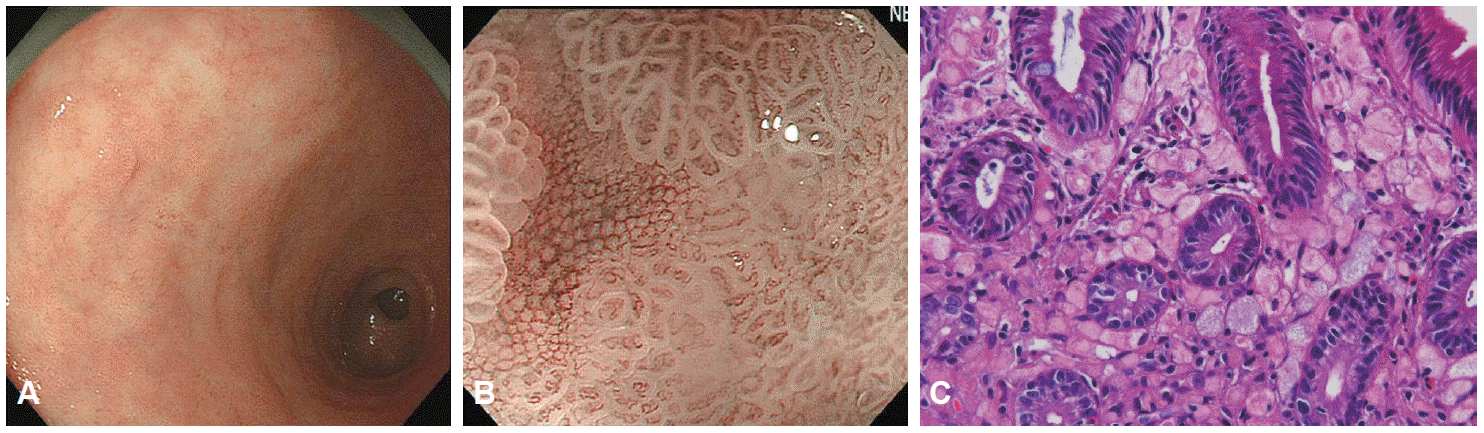
Fig. 8.
Usefulness of magnifying endoscopy with narrow-band imaging (M-NBI) for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (CE). (A) CE findings of a differentiated 0-IIb type early gastric cancer. The margins of lateral extent cannot be clearly delineated, let alone, the presence of cancer. (B) M-NBI findings of the oral margin of the cancer. Using M-NBI, a clear demarcation line (arrows) was identified. Within the demarcation line, an irregular microvascular pattern plus an absent microsurface pattern was present. Therefore, we successfully identified the cancer specific margin. (C) Because we determined the cancer-specific margins defining the cancer by M-NBI, we placed the markings just outside the demarcation lines according to the magnifying endoscopy using the M-NBI findings. Markings were traced by the white dotted line for the comparison with the following resected specimen after histopathological investigation. (D) The en bloc resected specimen by endoscopic submucosal dissection. According to the histopathological findings, the extent of the carcinoma (yellow lines) was reconstructed on the macroscopic photograph. The preoperative markings were clearly identified around the perimeter of carcinoma as shown by the white dotted line. Therefore, the lesion had been successfully delineated using M-NBI. Adapted from Nagahama et al., [23] with permission from Elsevier.
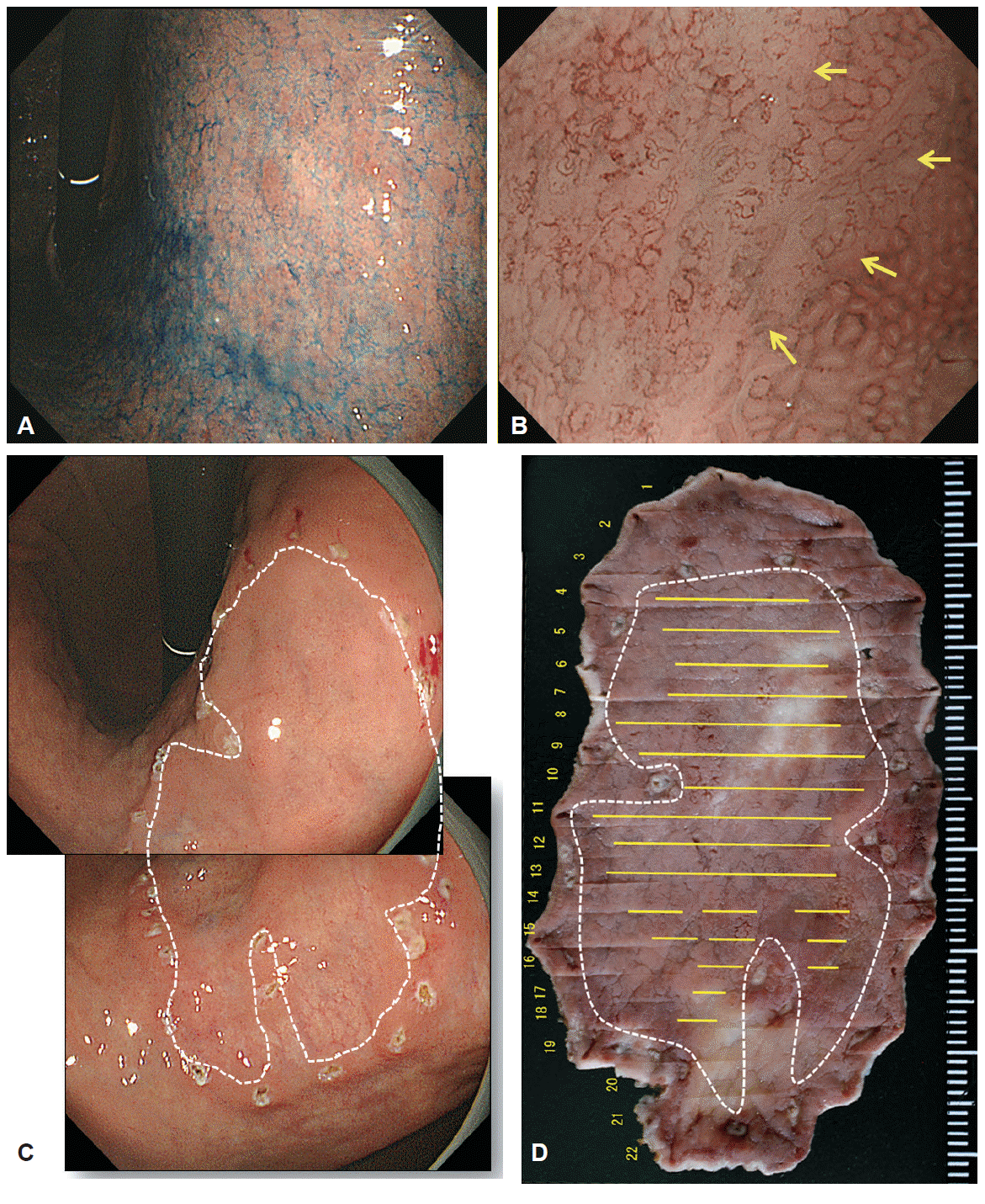
Fig. 9.
A strategy for determining the lateral extent of early gastric cancer for curative endoscopic resection. C-WLI, conventional white light imaging; CE, chromoendoscopy; M-NBI, magnifying endoscopy with narrow-band imaging.
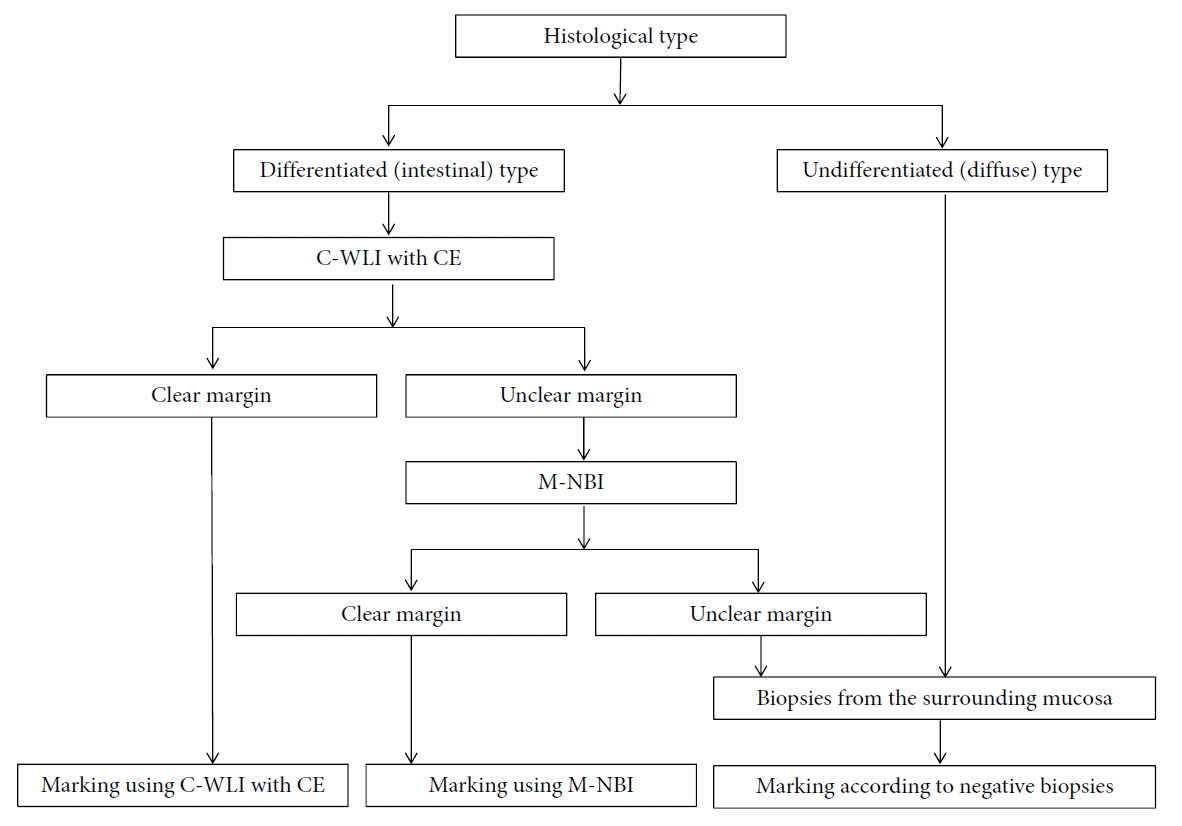
Table 1.
Diagnostic Performance of C-WLI and M-NBI for Gastric Small Depressed Lesions
| C-WLI (n=176) | M-NBI (n=177) | p-valuea) | |
|---|---|---|---|
| Accuracy, % | 65 (57–72) | 90 (85–94) | <0.001 |
| Sensitivity, % | 40 (19–64) | 60 (36–81) | 0.34 |
| Specificity, % | 68 (60–75) | 94 (89–97) | <0.001 |
Table 2.
Diagnostic Performance of C-WLI plus M-NBI for Gastric Small Depressed Lesions
| C-WLI (n=176) | C-WLI plus M-NBI (n=176) | p-valuea) | |
|---|---|---|---|
| Accuracy, % | 65 (57–72) | 97 (94–99) | <0.001 |
| Sensitivity, % | 40 (19–64) | 95 (75–100) | <0.001 |
| Specificity, % | 68 (60–75) | 97 (93–99) | <0.001 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download