Abstract
Most of subepithelial lesion (SEL) being identified was accidentally discovered as small bulging lesion covered with normal mucosa from endoscopic screening. The type of treatment and prognosis vary depending on the type of tumor, it would be crucial to perform an accurate differential diagnosis. Since the differentiation of SEL relied on the indirect findings observed from the mucosal surface using an endoscopy only in the past, it was able to confirm the presence of lesion only but difficult to identify complex detailed nature of the lesion. However, after the endoscopic ultrasonography (EUS) was introduced, it became possible to identify extrinsic compression, and size of intramural tumors, internal properties and contour so that it gets possible to have differential diagnosis of lesions and prediction on the lesion whether it is malignant or benign. In addition, the use of EUS-guided fine needle aspiration and EUS-guided core biopsy made it possible to make histological differential diagnosis. This study intended to investigate endoscopic and EUS findings, histological diagnosis, treatment regimen and impression of colorectal SELs.
Go to : 
Subepithelial lesions (SELs) of the gastrointestinal tract were previously known as submucosal tumors. However, they originate not only in the submucosa but also between the serosa layers in the deep mucosa; furthermore, they may not be a neoplastic lesion, but instead may be caused by compression of extrinsic structures. As a result, the term SEL is now widely used as it is considered to better reflect their nature. Colorectal SELs are mostly found in the appendix and rectum, but common lesions such as lipomas can be found in any part of the colon. The rapidly growing use of colonoscopy for purposes including health check-ups, cancer screening, and differential diagnosis of gastrointestinal symptoms, has led to an increasing number of SELs being detected. Most SELs are accidentally discovered during endoscopic screening and appear as a small bulging lesion covered with normal mucosa, with an incidence of about one in 300.1 The type of treatment and prognosis vary depending on the type of lesion, and some can become malignant, making an accurate differential diagnosis very important. A first step in achieving this is accurate endoscopic observation. The change in lesion should be observed through the postural variation of the patient and air volume control, as extrinsic compression may be caused by both normal and abnormal anatomical structures. In addition, a detailed observation of the size, shape, color, and position of the lesion, as well as the presence or absence of pulsatility, any accompanying abnormality of the mucosa, and rolling or pillow signs when the lesion is pushed with biopsy forceps are also important.
The detection of SEL previously relied only on indirect findings from the endoscopic observation of the mucosal surface, which allowed the presence of a lesion to be confirmed, but the complex, detailed nature of the lesion could often not be evaluated. However, after the introduction of endoscopic ultrasonography (EUS), it became possible to identify extrinsic compression, and the size of intramural tumors, their internal properties, contour, and primary layer, which in turns makes a differential diagnosis of lesions possible, including whether they are likely to be malignant. In addition, a pre-treatment EUS was used to determine whether endoscopic treatment was appropriate. These pre-treatment EUS evaluations provided useful information for therapeutic endoscopic target screening and for determining therapeutic effectiveness. Pre-treatment EUS allows the risk of perforation through the original layer from which the tumor was derived to be assessed, and the extent of vascular development inside the tumor, and hence also the risk of bleeding to be determined. It also allows the tumor growth pattern to be evaluated, which in turn makes it possible to assess whether endoscopic resection can be used when the tumor is of the dumbbell or extraluminal type. In addition, the use of EUS-guided fine needle aspiration (FNA) and EUS-guided core biopsy made it possible to make a histological differential diagnosis. Following on from this previous work, the aim of this review is to evaluate and summarize the endoscopic and EUS findings, histological diagnosis, treatment regimen, and appearance of colorectal subepithelial lesions, which are now more frequently found and are being actively studied.
Go to : 
Extramural compression can appear as changes in normal anatomical structures such as the cervix or prostate, an expanded intestine, pulsatile compression by serpentine vascular structures or endometriosis. In order to differentiate intramural lesions from extramural compression during endoscopic examination, the changes in the location and shape of the lesion should be observed while controlling the posture of the patient and the amount of air. If the shape of the lesion changes due to interlocking waves or the patient's posture, or the output and intake of the air through the endoscope, then the compression is highly likely to be extramural. Endoscopic observation allows intramural tumors and extramural compression to be distinguished with 89% accuracy, and the sensitivity for intramural lesions was as high as 98%, although the specificity was only 64%.2 It is therefore easy to diagnose intramural lesions through endoscopic observation but extramural lesions can be mistaken for intramural lesions in many cases. This suggests that other tests are needed, including EUS for the differential diagnosis of the SEL.
By investigating the relationship between the intestinal wall and the location of the tumor through EUS, it would be possible to more accurately differentiate between extramural compressions and intramural tumors. Specifically, when conducting EUS for a suspected SEL, any findings that surrounding organs or masses are pressing on the intestinal wall from outside the subserous layer (the fifth layer of the intestinal wall) would allow an extramural compression to be diagnosed. Conversely, a mass that was observed from the internal side of the intestinal wall could be diagnosed as a subepithelial tumor. This differentiation between extramural compression and SELs can be achieved accurately, with 92% sensitivity and 100% specificity.1
Go to : 
Detailed observation should be made of the size, shape, color, location, the presence of pulsatility, and any abnormality of the mucous surface of SELs that are often found during endoscopic examination. These findings are then used for differential diagnosis by combining them with the presence/absence of rolling or cushion signs. The mucosa covering the SEL usually appears normal, but in some cases, it can show redness, erosion, and ulceration. Erosion in the rectal mucosa indicates a carcinoid tumor, which may be accompanied by a depression of the central portion in larger lesions. Ulceration from the apex of the lesion is suggestive of submucosal carcinoma or lymphoma, a carcinoid tumor, or a gastrointestinal stromal tumor (GIST). More rarely, a distant metastasis of a malignant tumor originating in another organ, or direct infiltration by surrounding malignancies can form the same shape as an SEL, and in such cases, redness or ulceration of the surface mucosa is often present. In the case of lymphangioma, careful observation can reveal a shallow linear depression on the internal side of the lesion that is divided by a septa of fibrous connective tissue. The mucosa that covers the majority of the SEL appears to be the same color as the peripheral mucous in endoscopic observation, whilst lipomas and carcinoids appear yellow, and vascular lesions have a blue hue or appear pale blue, having a similar coloration to esophageal varices. Lymphangioma displays a pale smooth surface, appearing transparent with an off-white color in many cases. If the SEL can be depressed easily using biopsy forceps (positive for the pillow or cushion sign), it is highly likely to be a lipoma or a cyst. Lipomas are positive for the pillow sign and can be easily diagnosed by identifying the submucous fat tissues when the mucosa is removed using jumbo forceps.3 The internal portion of cysts is mostly filled with liquid, and the cysts itself has a transparent surface and feels soft when depressed with biopsy forceps. Simple cysts usually feel soft, but can feel hard if there is any fibrosis in the external wall. If the cyst is very soft, lymphangioma or a vascular abnormality should be considered. Lipomas that can be pushed from side to side using biopsy forceps (the rolling sign profile) are highly likely to be located under the muscularis mucosa, even if they are small. In addition, if their overall appearance is round or oval in shape, and they feel hard when pressed using biopsy forceps, a GIST, leiomyoma, or schwannoma originating from the muscularis propria should be considered. As with carcinoids, when at least part of the lesion is located in the lamina propria, or when the lesion is accompanied by fibrosis (such as an inflammatory fibroid polyp) making the margin difficult to distinguish from the normal mucosa, the lesion is considered to exhibit a negative rolling sign. If the lesion is small, it may not be visible upon endoscopy if there is an incorrect level of air infusion into the lumen, and the air volume should thus be adequately adjusted to measure the size of the lesion. Endoscopic measurements of lesions are often inaccurate and tend to overestimate their size. More accurate measurements can be achieved using a size indicator, but if a lesion is too small to allow this approach, it can be measured relatively accurately by comparing it to biopsy forceps when its mouth is completely opened. The location of the lesion can also be useful in its differential diagnosis. Lipoma arises in the right colon, especially around the ileocecal valve. It is not observed within the appendiceal opening, but if an SEL is present at this location, the possibility of an appendiceal mucocele should be considered. Furthermore, lymphangioma frequently arises in the colon, whereas carcinoids are more often present in the rectum.
If the aforementioned endoscopic findings are sufficient to diagnose a lipoma or cyst when considered collectively, further examination is not necessary. However, if a lesion is accompanied by erosions or ulcers, or if it has a yellowish tint and is solid or firmly attached to the submucosa and larger than 1 cm, additional diagnostic tests will be needed.
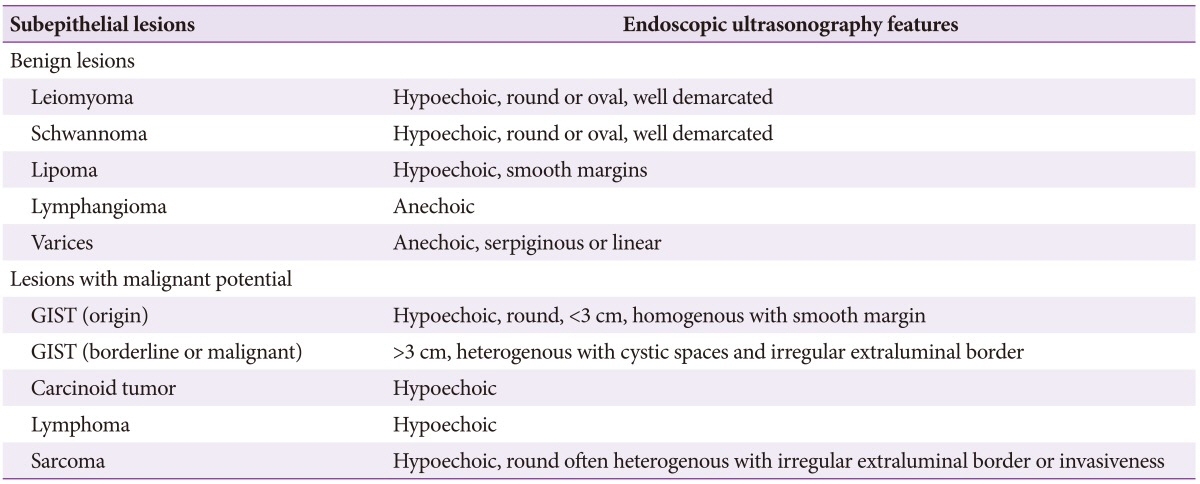
When SEL is found during colonoscopy and it is determined that further examination is required after the careful endoscopic observation described above, a EUS should be conducted. SELs of the upper gastrointestinal tract or rectum can be examined using the EUS of forward-viewing endoscopy and a catheter probe, but for the right side of the colon, EUS of forward-viewing endoscopy has a tip that is too hard to pass the narrow or curved part of the colon and could cause complications such as bleeding or perforation. Thus, for examination of these parts of the colon, a mounted catheter probe is useful.4 However, it is still difficult to determine the overall appearance or starting point of large lesions using catheter probe mounted EUS. In addition, when the lesion cannot be located because water is retained along the gravity direction, examination itself is impossible. To overcome these difficulties, the recently developed frontal lens-assisted linear array EUS is expected to be helpful in the future.5 EUS is the most accurate method to determine the characteristics of SEL.6 It allows the intramural or extramural location of a tumor to be determined, and, if its location is intramural, the identification of the layer from which the lesion originated. This information helps to determine the correct diagnosis.3 The positional relationship between the lesion and the intestinal wall should initially be determined by EUS, and if extramural compression is present, the role of any anatomical abnormalities should then also be determined. For intramural tumors, the lesion should first be measured from the nearest distance as accurately as possible. Next, the appearance of the lesion and its margin should be noted, and then the homogeneity, echogenicity, and echotexture of the inner structure and inner tissues should be observed in order to allow a presumptive diagnosis. For anechoic lesions, a Doppler test should be conducted to determine the degree of tumor vascularization and blood flow, the presence of which indicates a cystic lesion. Information can also be collected about whether the margin of the lesion is clear, whether the outer edge is smooth, soft, and continuous, or whether the surrounding structures have been transformed due to a nodular or irregular peripheral outer margin. Based on these EUS observations, it is possible to narrow the range of possible diagnoses, and to diagnose cysts or lipomas without any further tests including biopsy. However, the reported agreement between overall presumptive diagnoses based on EUS findings and biopsy results remains unsatisfactory, at about 43% to 79%.27 Around 13% of subepithelial tumors of the gastrointestinal tract are malignant (range, 0% to 27%),89 although unfortunately the most important objective of EUS-the noninvasive diagnosis of malignant tumors-is yet to be achieved. Findings suggestive of malignancy are a large tumor sized ≥3 cm, an irregular margin, the presence of a hyperechoic or cystic lesion or a heterogeneous echo, and the presence of ulcers or lymphadenopathy. Of these, tumor size is the most useful diagnostic finding. In addition, rapid tumor growth apparent at the follow-up examination is usually regarded as being indicative of malignancy.
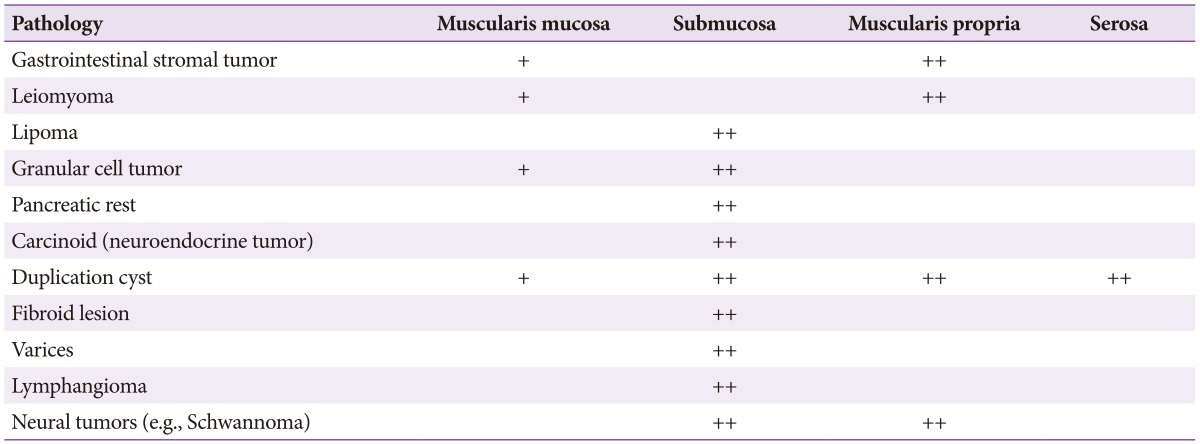
Mesenchymal tumors classified as leiomyoma, GIST, or schwannoma are difficult to differentiate on the basis of endoscopic and EUS findings, and in these cases a biopsy is required followed by pathological evaluation with specific stains (Tables 1, 2).
These usually appear as uniform hypoechoic lesions with a homogenous margin originating from the second layer or the muscularis propria, although they may also appear as a pedunculated or multi-lobulated structure on EUS. Microechogenic dots or calcification-induced intense echogenic dots are also relatively common.
GISTs of the large intestine often become malignant and appear as hypoechoic lesions originating from the muscularis propria. Malignancy is suggested by an internal cystic change of at least 3 cm with an irregular outer margin accompanied by lymphadenopathy.
These are usually found in the colon and have a soft appearance with a yellow tint upon endoscopic examination. When pressed with closed biopsy forceps, these lesions exhibit pillow or cushion signs as the center can be depressed deeply. Lipoma can be diagnosed easily due to its characteristic findings in EUS and endoscopic treatment can be performed based on these findings.10
These are typically found in the gastrointestinal tract, mainly in the rectum, appear yellow in endoscopic evaluation and may contain a central depression. Carcinoids are typically observed as homogeneous, hypoechoic masses with distinct margins that originate from the second layer and invade the submucosal layer. They are usually sized ≤2 cm as the submucosal layer is well maintained. If there is no distant metastasis, they can be removed by endoscopic resection.11
These are apparent as anechoic lesions in the submucosal layer by EUS and many of them have an internal septum. Mucocele, in particular appendiceal mucocele, is observed in EUS as a hypoechoic extramural tumor compressing the appendix, and has microhyperechogenic dots internally.12
These form large polyps. The mucosa covering varices are thickened so it can be difficult to distinguish them endoscopically from other SELs, and, if conducting a tissue biopsy for differentiation, care is needed to avoid massive bleeding. In EUS, rectal varices are situated in the submucosal layer, and have a tortuous and anechoic appearance. Perirectal collaterals around the rectum are also identifiable.13
When endometriosis invades the intestines, it is mainly apparent as an SEL from the sigmoid colon and rectum. EUS usually shows it to be located in the muscularis propria, and reveals it to have a fusiform or semilunar form. It is observed as a homogenous or inhomogeneous hypoechoic lesion, accompanied by an internal cystic echo.1415 In general, it is difficult to distinguish from a myogenic tumor and it invades the submucosal layer in about 40%.1516
Go to : 
It is usually difficult to decide when and how to perform a tissue biopsy for SEL. Given the low accuracy of endoscopic or EUS-based diagnosis, histological diagnosis remains the most accurate diagnostic method for SELs, but it is difficult to obtain an adequate tissue sample from the endoscopic mucosa biopsy alone, and other methods are technically difficult and can led to complications. Therefore, it needs to be carefully considered whether or not the observed SEL requires histological diagnosis. As described above, lipomas or cysts do not require histological diagnosis and if the tumor is a vascular lesion, the biopsy can cause fatal complications. Furthermore, in situations that require surgical resection, for example, when the lesion is large or causes symptoms, preoperative biopsy may not be particularly helpful. The most important issue in clinical practice is the differential diagnosis of SEL, in particular whether the lesion is non-cancerous, cancerous, or precancerous. Therefore, if a hypoechoic mass is found to originate in the third or fourth layer by EUS, histological diagnosis is usually considered. When the lesion is small and is not examined using EUS, but is considered to require histological diagnosis, a forceps biopsy can be performed. If the tissue sample can be obtained from the lower layer by pressing the forceps more deeply, unlike an ordinary biopsy using forceps that mainly obtains tissues from the epithelial layer and the muscularis propria, it is possible to diagnose myoma originating from the muscularis mucosae and carcinoids of the deep mucosa. When there is ulceration or erosion in the center, a biopsy increases the diagnostic possibilities. In addition to the above, the histological diagnosis of SEL includes the following.
(1) Repeated biopsy: repeated biopsy using a large biopsy forceps and sampling the same site is referred to as a tunneled, stacked forceps, or bite-on-bite biopsy. Its implementation is simple but the possibility of complications such as bleeding and perforation should be considered and indeed have been reported in approximately 17% to 42% of cases, requiring hospitalization in 2.8% of cases.1718
(2) EUS-FNA cytology or EUS-FNA biopsy: EUS-FNA can help in the differentiation of SEL. However, compared to EUS-FNA diagnosis of lymph node or pancreatic lesions, the diagnostic accuracy of SEL by EUS-FNA is somewhat low, at 60% to 90%.1920 Since EUS-FNA samples provide only limited material for the immunohistochemistry analysis required for diagnosing GIST or counting mitotic cells for the determination of malignancy, EUS-guided core biopsy or EUS-guided Trucut biopsy are conducted instead. Nevertheless, since the insertion of the endoscope itself is difficult other than through rectum, and due to severe flexion of the endoscope, it is impossible to perform EUS-FNA or EUS-guided Trucut biopsy. In addition, a EUS-guided core biopsy cannot be performed, even if the size of the lesion does not exceed 2 cm.
(3) Endoscopic submucosal dissection (ESD): ESD can be performed for both the histological diagnosis of SEL and therapeutic resection. Although the diagnostic value of ESD is superior to that of the aforementioned repeated biopsy, greater caution is needed to avoid complications due to perforation because lower gastrointestinal tract tumors have thinner walls than their upper gastrointestinal tract counterparts. Consequently, ESD has been mainly performed for SELs confined to the deep mucosa and submucosa. However, there have been recent reports of successful resections even in cases of tumors originating in the muscularis propria, made possible by advances in various endoscopic techniques. However, in cases of tumors originating from the muscularis propria in which significant malignancy remains after resection, additional surgical resection may be required. This in turn increases the risk of perforation and the associated tumor spillage. Major complications other than perforation have been reported, including bleeding in approximately 3% to 9% of ESDs, and special attention may also be needed in these cases.1121
When the lesion is smaller than 1 cm and its location precludes EUS, and when histological diagnosis is difficult to conduct due to the danger of complications, follow-up endoscopy can be considered. Furthermore, a follow-up EUS can be performed if there are no findings such as a hypoechoic mass, an irregular margin, a cystic space, or malignant lymph nodes that are suggestive of a malignant GIST situated in the muscularis propria. However, before deciding on the follow-up observation, patients must be consulted regarding its dangers and the pros and cons of possible alternatives, such as histological examination and surgical resection that uses the aforementioned methods. For endoscopic or EUS follow-up observation, the appropriate intervals have yet to be established, and planning requires an individualized approach taking into account factors such as the patient's age and health, and the malignant potential of the lesions. Currently, EUS is generally performed with a 1-year interval and if there is no change in the first 2 years then this interval is increased to 2 years.
Go to : 
SELs are found incidentally in around 1% of routine endoscopic examinations, although this is increasing with the growing use of colonoscopy for colon cancer screening. In some rare cases, the lesion is found to be malignant, which requires appropriate care. Close endoscopic observation alone provides significant information about the lesion, and in some cases that is sufficient to allow a differential diagnosis. EUS is the best method for the differential diagnosis of SEL, but provides only a low degree of diagnostic specificity. Biopsy may be performed using a variety of methods to allow a more accurate diagnosis, but the necessity and safety of this approach must be considered with respect to endoscopic and EUS findings. There is no established method for follow-up of these patients, but if the lesion increases in size or changes in internal echo are identified by EUS, then more aggressive treatments such as endoscopic or surgical resection should be considered. If future studies of each disease-specific prognosis are conducted and EUS technology improves, then it is seems highly likely that the role of EUS follow-up will be extended.
Go to : 
References
1. Landi B, Palazzo L. The role of endosonography in submucosal tumours. Best Pract Res Clin Gastroenterol. 2009; 23:679–701. PMID: 19744633.

2. Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005; 62:202–208. PMID: 16046979.

3. Menon L, Buscaglia JM. Endoscopic approach to subepithelial lesions. Therap Adv Gastroenterol. 2014; 7:123–130.

4. Chen TH, Lin CJ, Wu RC, et al. The application of miniprobe ultrasonography in the diagnosis of colorectal subepithelial lesions. Chang Gung Med J. 2010; 33:380–388. PMID: 20804667.
5. Nguyen-Tang T, Shah JN, Sanchez-Yague A, Binmoeller KF. Use of the front-view forward-array echoendoscope to evaluate right colonic subepithelial lesions. Gastrointest Endosc. 2010; 72:606–610. PMID: 20561620.

6. Kim EY. Endoscopic ultrasound, where are we now in 2012? Clin Endosc. 2012; 45:321–323. PMID: 22977827.

7. Kwon JG, Kim EY, Kim YS, et al. Accuracy of endoscopic ultrasonographic impression compared with pathologic diagnosis in gastrointestinal submucosal tumors. Korean J Gastroenterol. 2005; 45:88–96. PMID: 15725712.
8. Polkowski M, Butruk E. Submucosal lesions. Gastrointest Endosc Clin N Am. 2005; 15:33–54. PMID: 15555950.

9. Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005; 37:635–645. PMID: 16010608.

10. Kibria R, Khalil Q, Siraj U, Ali SA, Akram S. Giant ulcerated lipoma of the colon causing iron deficiency anemia successfully treated with endoscopic ultrasound-assisted resection. South Med J. 2009; 102:1058–1060. PMID: 19738527.

11. Ishii N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc. 2010; 24:1413–1419. PMID: 20033710.

12. Uradomo LT, Darwin PE. Evaluation of subepithelial abnormalities of the appendix by endoscopic ultrasound. Diagn Ther Endosc. 2009; 2009:295379. PMID: 19920863.

13. Yasuda K, Cho E, Nakajima M, Kawai K. Diagnosis of submucosal lesions of the upper gastrointestinal tract by endoscopic ultrasonography. Gastrointest Endosc. 1990; 36(2 Suppl):S17–S20. PMID: 2184080.

14. Pishvaian AC, Ahlawat SK, Garvin D, Haddad NG. Role of EUS and EUS-guided FNA in the diagnosis of symptomatic rectosigmoid endometriosis. Gastrointest Endosc. 2006; 63:331–335. PMID: 16427951.

15. Roseau G, Dumontier I, Palazzo L, et al. Rectosigmoid endometriosis: endoscopic ultrasound features and clinical implications. Endoscopy. 2000; 32:525–530. PMID: 10917184.

16. Nickl N. Endoscopic approach to gastrointestinal stromal tumors. Gastrointest Endosc Clin N Am. 2005; 15:455–466. PMID: 15990051.

17. Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc. 2003; 57:68–72. PMID: 12518134.

18. Cantor MJ, Davila RE, Faigel DO. Yield of tissue sampling for subepithelial lesions evaluated by EUS: a comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest Endosc. 2006; 64:29–34. PMID: 16813799.

19. Jenssen C, Dietrich CF. Endoscopic ultrasound-guided fine-needle aspiration biopsy and trucut biopsy in gastroenterology: an overview. Best Pract Res Clin Gastroenterol. 2009; 23:743–759. PMID: 19744637.
20. Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009; 69:1218–1223. PMID: 19394006.

21. Park HW, Byeon JS, Park YS, et al. Endoscopic submucosal dissection for treatment of rectal carcinoid tumors. Gastrointest Endosc. 2010; 72:143–149. PMID: 20381798.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download