Abstract
Biliary-enteric communications caused by duodenal ulcers are uncommon, and choledochoduodenal fistula (CDF) is by far the most common type. Usually in this situation, food material does not enter the common bile duct because the duodenal lumen is intact. Here, we report a case in which cholangitis occurred due to food materials impacted through a CDF. Duodenal obstruction secondary to duodenal ulcer prevented food passage into the duodenum in this case. Surgical management was recommended; however, the patient refused surgery because of poor general condition. Consequently, the patient expired with sepsis secondary to ascending cholangitis.
Spontaneous biliary-enteric fistulas are usually caused by choledocholithiasis when stones perforate through the inflamed biliary tree into an otherwise normal duodenum.1,2 Biliary-enteric fistulas secondary to duodenal ulcer are rare, and choledochoduodenal fistula (CDF) is the most common type.2 Because the duodenal lumen is intact in most cases, food contents usually do not enter the fistulous tract, and complications such as cholangitis and obstructive jaundice usually do not occur. However, such complications may occur if the drainage of gastric contents is inadequate and food material regurgitates through the fistula.3 Here, we report a case of ascending cholangitis with obstructive jaundice secondary to food materials impacted in the common bile duct (CBD) through a CDF.
An 82-year-old woman visited our emergency room because of epigastric pain for the previous 3 days. She suffered from degenerative joint disease and had consumed large amounts of various nonsteroidal anti-inflammatory drugs (NSAIDs) for the preceding several months. Thirteen years before, she had undergone a cholecystectomy for treatment of gallbladder stones. Laboratory tests revealed the following levels: white blood cell count, 7.17×109/L (reference range, 4.0 to 10.0); hemoglobin, 8.1 g/dL (reference range, 12 to 16); total bilirubin 10.5 mg/dL (reference range, 0.2 to 1.2); direct bilirubin 5.8 mg/dL (reference range, 0 to 0.3); aspartate aminotransferase 119 IU/L (reference range, 0 to 40); alanine aminotransferase 84 IU/L (reference range, 0 to 40); alkaline phosphatase, 720 IU/L (reference range, 39 to 117); and γ-glutamyl transpeptidase, 561 (5 to 55 IU/L).
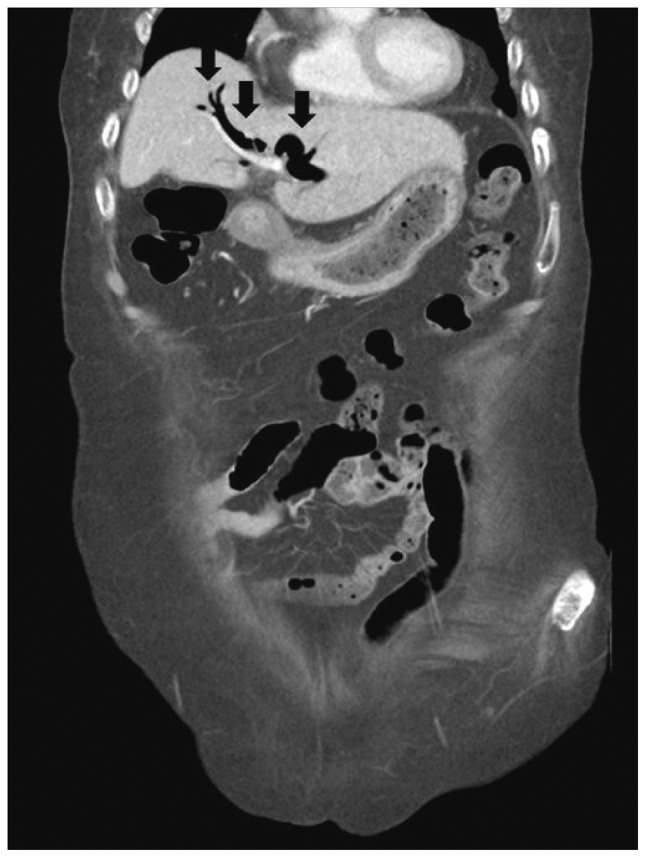
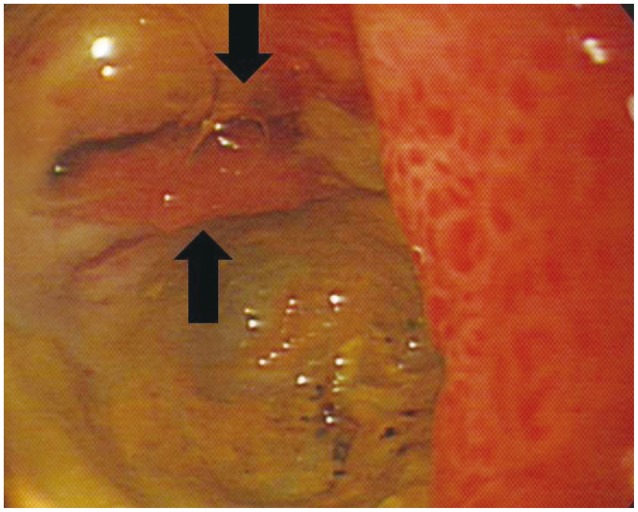
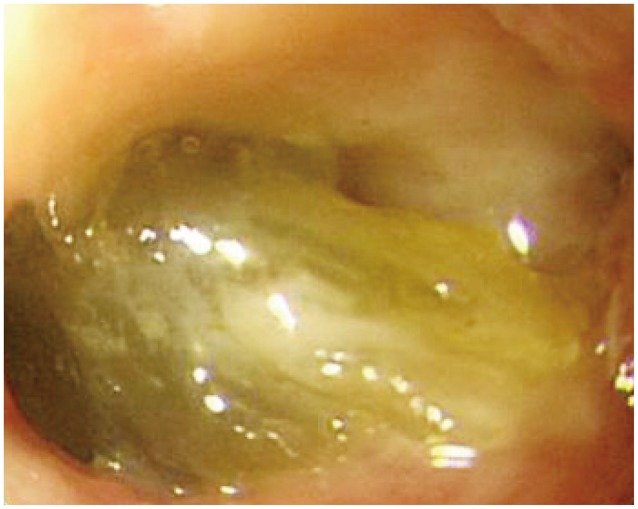
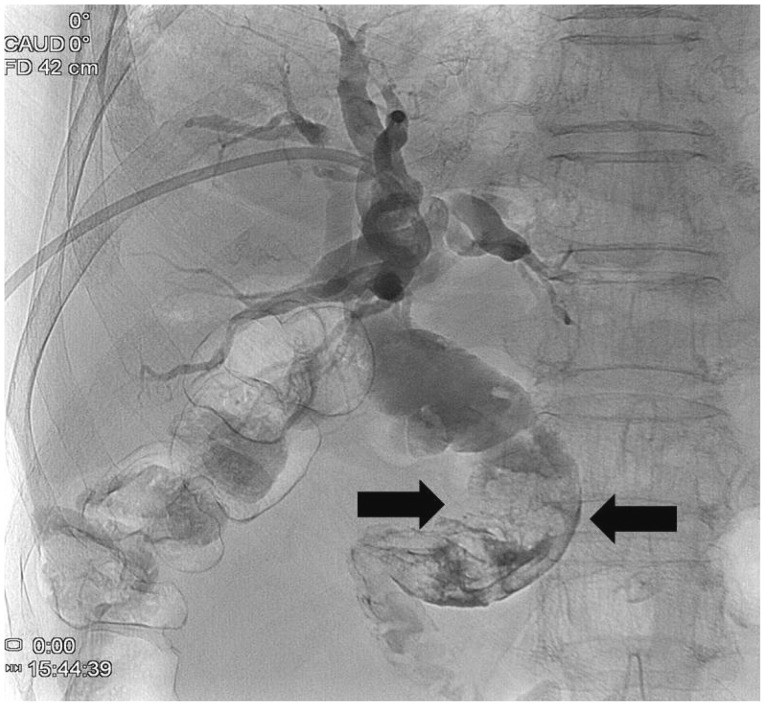
Abdominal computed tomography (Fig. 1) showed dilatation of intra- and extra-hepatic bile ducts with pneumobilia but did not show any choledocholithiasis. The following day, esophagogastroduodenoscopy (EGD) revealed an active duodenal ulcer with stenosis, and the endoscope was not able to pass through the duodenal lumen. The orifice of a CDF was noted on the anterior wall side of the duodenal bulb (Fig. 2). On follow-up EGD, food material was impacted at the orifice of the CDF (Fig. 3), suggesting that food material had passed into the CBD. The material was removed with endoscopic forceps. A percutaneous transhepatic bile drainage (PTBD) catheter was inserted into the CBD, and tubography showed food impaction with CBD dilatation (Fig. 4). After PTBD insertion, total bilirubin levels steadily decreased. To resolve the stenosis of the duodenal bulb and to seal the CDF, a partially covered self-expendable metallic stent (Hanarostent, duodenal/pyloric; M.I. Tech Co., Ltd., Seoul, Korea) was placed in the duodenum. The CDF was closed, and the stent was removed 6 weeks after insertion. Since impacted food material was still in the CBD, endoscopic retrograde cholangiography was performed to remove the food material; however, this was not successful because of the deformity of the duodenal bulb. We then recommended surgical management, but the patient refused because of poor general condition. The PTBD catheter was left in place, and medical therapy, including proton pump inhibitor and PTBD, was prescribed.
Three months after her discharge from the hospital, the patient expired at a local health care facility from sepsis secondary to ascending cholangitis.
The unique point of this case is that food material impaction in the CBD through a CDF caused cholangitis with obstructive jaundice. It is plausible that the food material passed through the fistula tract because duodenal obstruction secondary to duodenal ulcer prevented normal food passage through the duodenum. Although we initially thought that the CDF was a result of NSAID-associated duodenal ulcer, the possibility of choledocholithiasis as the cause should also be considered since this patient had a history of cholecystectomy for treatment of gallbladder stones.
Spontaneous biliary-enteric fistulas caused by primary duodenal diseases are uncommon.2 Their rarity derives from the fact that duodenal ulcers typically occur within 4 cm distal to the pylorus, whereas the CBD is about 7 cm distal to the pylorus.4 Nevertheless, approximately 75% of biliary-enteric fistulas secondary to ulcer diseases are CDFs.2 Extensive fibrosis and adhesions, particularly of the hepatogastric and hepatoduodenal ligaments, could easily alter the normal anatomical relationship and make this possible.5
The usual symptoms in CDF due to duodenal ulcer are those of acid-peptic disease. No specific signs or symptoms strongly imply the presence of CDF, and the patient's complaints stem from the primary disease process, such as duodenal ulcer.4,6 In our patient, the duodenal ulcer was thought to be a result of long-term consumption of NSAIDs.
Biliary-enteric fistulas are usually diagnosed on radiography incidentally because they seldom produce clinical symptoms.4 Radiography may show the presence of air in the biliary tract or gallbladder. Pneumobilia occurs in only 14% to 58% of patients with CDF, and although helpful when present, its absence cannot exclude the diagnosis.7 In our patient, abdominal computed tomography revealed pneumobilia and did not show gallstones or CBD stones. Abdominal sonography may show air in the biliary tree and is very helpful for differentiating a biliary-enteric fistula secondary to gallstones from one caused by CBD stones. A barium imaging study may show a fistulous tract connecting the duodenum with the biliary tree.7,8 Demonstration of an orifice discharging bile during endoscopy is the most reliable sign of a CDF. Endoscopic retrograde cholangiography via the fistula orifice has been reported.1,6,9 In our patient, the fistula orifice in the duodenal bulb was demonstrated during endoscopy, and injection of contrast into the orifice showed contrast filling in the biliary tree.
The treatment of CDF is first to treat the underlying peptic ulcer. Medical management is advocated when possible, and surgery unnecessary if the patient is symptom-free.3,10 Healing of the ulcer is accompanied in most cases by healing of the fistula.1,2 Closure of the CDF with medical management can be achieved in most cases. Surgery is needed for patients who have poorly controlled or excessively recurrent ulcer symptoms, major complications of ulcer such as hemorrhage, or, in the exceptional patient, obstruction with cholangitis or biliary obstruction.1,7,11 Our patient had an obstruction due to a duodenal ulcer, which led to food impaction in the CBD. Ascending cholangitis developed from the impacted food material, and surgery was mandatory. However, surgery was not performed because of refusal by the patient.
In summary, we have described a case of ascending cholangitis with obstructive jaundice secondary to food material impacted in the CBD through a CDF. This has never before been reported in the English literature. Physicians should be aware of this rare complication.
References
1. Feller ER, Warshaw AL, Schapiro RH. Observations on management of choledochoduodenal fistula due to penetrating peptic ulcer. Gastroenterology. 1980; 78:126–131. PMID: 7350019.

2. Sarr MG, Shepard AJ, Zuidema GD. Choledochoduodenal fistula: an unusual complication of duodenal ulcer disease. Am J Surg. 1981; 141:736–740. PMID: 7246867.

3. Topal U, Savci G, Sadikoglu MY, Tuncel E. Choledochoduodenal fistula secondary to duodenal peptic ulcer. A case report. Acta Radiol. 1997; 38:1007–1009. PMID: 9394658.
4. Michowitz M, Farago C, Lazarovici I, Solowiejczyk M. Choledochoduodenal fistula: a rare complication of duodenal ulcer. Am J Gastroenterol. 1984; 79:416–420. PMID: 6720662.
5. Lewis EA, Bohrer SP. Choledochoduodenal fistula complicating chronic duodenal ulcer in Nigerians. Gut. 1969; 10:146–149. PMID: 5766044.

6. Naga M, Mogawer MS. Choledochoduodenal fistula: a rare sequel of duodenal ulcer. Endoscopy. 1991; 23:307–308. PMID: 1743142.

7. Jaballah S, Sabri Y, Karim S. Choledochoduodenal fistula due to duodenal peptic ulcer. Dig Dis Sci. 2001; 46:2475–2479. PMID: 11713956.
8. H'Ng MW, Yim HB. Spontaneous choledochoduodenal fistula secondary to long-standing ulcer disease. Singapore Med J. 2003; 44:205–207. PMID: 12952034.
9. Wong WM, Hu WH, Lai KC. Images of interest. Hepatobiliary and pancreatic: choledochoduodenal fistula secondary to duodenal ulcer disease. J Gastroenterol Hepatol. 2004; 19:829. PMID: 15209635.
10. Page JE, Dow J, Dundas DD. Ulcerogenic choledochoduodenal fistula. Clin Radiol. 1989; 40:58–60. PMID: 2920522.

11. Fowler CL, Sternquist JC. Choledochoduodenal fistula: a rare complication of peptic ulcer disease. Am J Gastroenterol. 1987; 82:269–271. PMID: 3826035.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download