Abstract
The Japanese Classification of Gastric Carcinoma histologically classifies endoscopically resected gastric cancer into differentiated and undifferentiated types according to the presence or absence of tubular structures on histology. The former includes papillary adenocarcinoma and tubular types, and the latter includes poorly differentiated adenocarcinoma, signet ring cell carcinoma and mucinous adenocarcinoma. However, gastric cancer sometimes contains a mixture of differentiated and undifferentiated components, and the clinical outcomes of the histological mixture are unknown, especially following endoscopic resection of early gastric cancer (EGC). This case was within the guideline indications for endoscopic submucosal resection (ESD), although it contained a partly signet ring cell carcinoma component; it recurred after 19 months with multiple lymph node and liver metastases. This case shows that additional surgical resection after ESD should be performed for patients with any mixed signet ring cell component, even in mild or moderately differentiated EGC.
Go to : 
Endoscopic resection for early gastric cancer (EGC) with no risk of lymph node metastasis is currently the standard treatment in Korea and Japan, and is gaining worldwide acceptance.1,2 Endoscopic submucosal dissection (ESD) has the major advantage of enabling en bloc resection irrespective of tumor size, which enables exact pathological examination compared to conventional endoscopic mucosal resection.3 The extended indications for endoscopic resection of EGC without lymphovascular invasion are as follows: (1) intramucosal differentiated adenocarcinoma without ulceration regardless of lesion size; (2) intramucosal differentiated adenocarcinoma with ulceration ≤30 mm in diameter; (3) submucosal invasion 1 (SM1) differentiated adenocarcinoma ≤30 mm in diameter; and (4) intramucosal undifferentiated adenocarcinoma without ulceration ≤20 mm in diameter.4 In some cases, gastric cancer includes a mixture of differentiated and undifferentiated components.5 The Japanese Classification of Gastric Carcinoma classifies the mixed histological type based on the predominant histological component.5 However, little is known about the clinical significance of the histological mixture, especially with regards to endoscopic resection. We report a case of early liver metastasis after ESD in a predominantly differentiated type of EGC with a signet ring cell component.
Go to : 
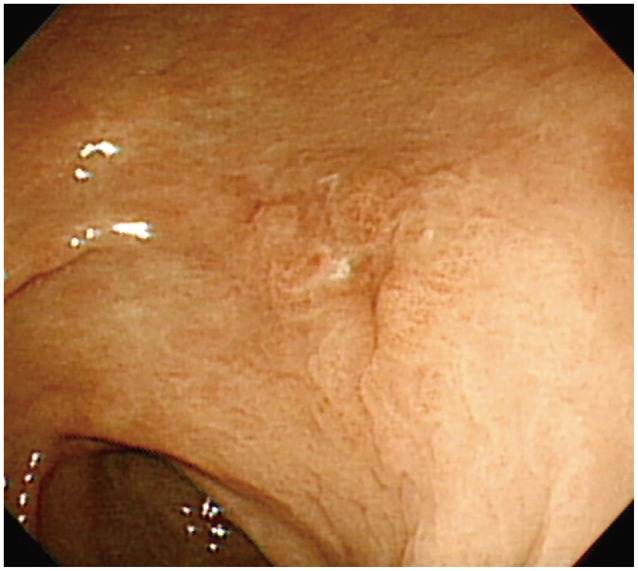
A 73-year-old man underwent esophagogastroduodenoscopy (EGD) for epigastric discomfort. This revealed a 1.5-cm superficial elevated lesion with a central depression on the posterior wall of the mid-antrum (Fig. 1). The pathology was well-differentiated adenocarcinoma. There was no demonstrable stomach mass, perigastric fat infiltration, or regional lymph node metastasis on abdomen pelvic computed tomography (CT). Therefore, ESD was proposed because the lesion was small (1.5 cm), and the histology grossly suggested a diagnosis of intramucosal cancer of differentiated type.
The specimen measured 4.6×4.3 cm and the cancer lesion located in the center of the specimen measured 1.5×1.0 cm. It was resected en bloc without any immediate complications (Fig. 2). The histopathology showed a moderately differentiated intestinal type of adenocarcinoma, with a signet ring cell component (15% to 20%), EGC type 0-IIa+IIc (1.5×1.2×0.2 cm). It was confined to the muscularis mucosa, with no lymphovascular invasion or ulceration. There was a clear lateral and vertical resection margin (Fig. 3).
We suggested that the patient be followed closely or that he undergo additional surgical resection because of the signet ring cell component. The patient preferred to be followed closely due to his age and other medical problems. We repeated the EGD at 1, 4, 7, and 13 months, with CT at 7 and 13 months. There was no evidence of tumor recurrence in these examinations.
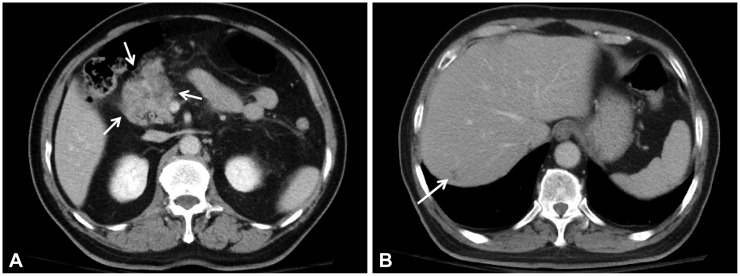
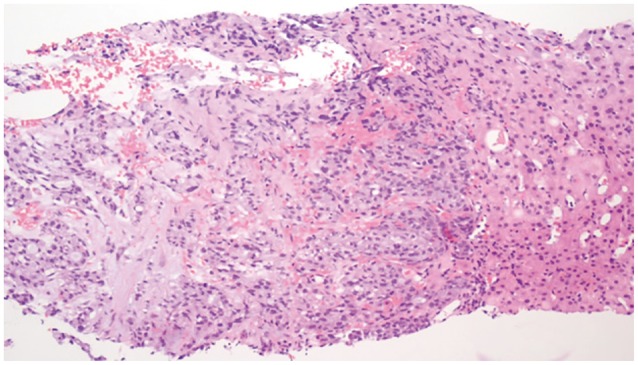
Nineteen months after the initial ESD, the patient presented to the Emergency Department with continuous abdominal pain. Liver function test were all within normal limits. Serum amylase and lipase levels increased to 275 and 1,090 U/L, respectively. Serum α-fetoprotein and cancer antigen 19-9 levels were 1.9 and 11.5 IU/mL. Serologic testing revealed negative results for hepatitis B surface antigen and hepatitis C virus antibody. Abdominopelvic CT showed a 5×3-cm mass with a central necrotizing portion between the pre-pyloric antrum and the pancreas head. Multiple enlarged regional lymph nodes were seen around the peripancreatic mass. The mass had invaded the pancreas head directly, causing pancreatitis, which appeared as swelling of the pancreas and peripancreatic infiltration. A new 1.5 cm, ill-defined, low-density lesion was seen in segment 7 of the liver (Fig. 4). There was no evidence of local recurrence on EGD or endoscopic biopsy. Positron emission tomography-CT showed hypermetabolic activity in the area between the pre-pyloric antrum and the pancreas head, but isometabolic activity in segment 7 of the liver. An ultrasound-guided core biopsy of the liver mass was done to rule out liver metastasis. The mass was identified as a moderately differentiated adenocarcinoma with signet ring cell features compatible with liver metastasis of EGC (Fig. 5). The patient underwent chemotherapy for recurrent gastric cancer with liver metastasis.
Go to : 
Gastric cancer can be classified as differentiated and undifferentiated types according to the presence or absence of tubular structures on histology as stated in the Japanese Classification of Gastric Carcinoma.5 Undifferentiated gastric cancer has a higher risk of lymph node metastasis than does differentiated gastric cancer.4 Therefore, the histological type is one indicator for endoscopic resection of EGC.4 However, gastric cancer sometimes contains a mixture of differentiated and undifferentiated components, and the clinical outcomes of the histological mixture are not known, especially following endoscopic resection of EGC. The Japanese Classification of Gastric Carcinoma classifies gastric cancer that includes a mixture of differentiated and undifferentiated components according to the predominant histological type.5 Gotoda et al.4 proposed extended indications for ESD in EGC based on whether the type was differentiated or not. If a predominantly differentiated EGC with some undifferentiated components is classified as differentiated gastric cancer using the Japanese classification and meets the extended criteria of ESD, the lesion can be followed without additional surgical resection. In our case, the EGC was classified as differentiated adenocarcinoma according to the Japanese classification based on the predominantly moderately differentiated type on histopathology, although it contained a signet ring cell component (15% to 20%). Since it was a differentiated carcinoma, measuring 1.5 cm, with no ulceration, and confined to the muscularis mucosa, it can be considered for curative resection according to the guideline indications. Iwamoto et al.6 reported that a mixed type EGC had more submucosal invasion than a pure differentiated type in a patient treated with ESD. However, there were no recurrences or metastases in any of the seven mixed type cases. Shimizu et al.7 reported that differentiated predominantly mixed type EGC was more likely to have lymphatic invasion and lymph node metastases, and a significantly poorer 5-year survival than the pure differentiated type. Undifferentiated mixed type EGC had more incomplete resection and lateral margin invasion than pure undifferentiated type EGC after ESD, although the difference was not statistically significant.8 Consequently, the different behaviors of mixed type EGC compared with pure differentiated or undifferentiated EGC is consistent with the development of early liver metastasis after ESD as seen in our case.
In this case, a diagnosis of pancreatic head cancer with liver metastasis may be considered. However, primary signet ring cell carcinoma of the pancreas is extremely rare, with only four cases reported in the literature.9 We therefore differentiated EGC with liver and peripancreatic metastasis from pancreatic head cancer with liver metastasis. The radiologic findings, which showed a mass with a central necrotizing portion, multiple enlarged regional lymph nodes around the peripancreatic mass, and the peripancreatic mass occupying a bigger portion than pancreatic parenchymal lesion, support the diagnosis of EGC with liver and peripancreatic metastasis but not that of primary pancreatic cancer.
As the evidence is still insufficient for the best treatment of differentiated tumors associated with some areas of undifferentiated histology, The Japanese Gastric Cancer Treatment Guidelines 2010 recommend additional surgical treatment for differentiated intramucosal cancer with areas of undifferentiated-type carcinoma that exceed 2 cm or tumor size ≤3 cm, differentiated intramucosal cancer with ulceration, and any component of undifferentiated-type carcinoma.10 However, as our case did not meet these criteria, the resection was considered curative. Recently, Huh et al.11 reported that EGC with signet ring cell mixed histology behaved more aggressively than other histologies in patients undergoing surgical resection for EGC. The mixed signet ring cell carcinoma was associated with more submucosal invasion, larger size, higher lymph node metastasis than signet ring cell carcinoma, a well or moderately differentiated adenocarcinoma, and a poorly differentiated adenocarcinoma. The mixed signet ring cell carcinoma was one of the independent risk factors of lymph node metastasis. Therefore, they recommended additional surgery after endoscopic resection or lymph node dissection for minimally invasive surgery in EGC with signet ring cell histology. As our case also had a signet ring cell component and metastasized to the liver early during follow-up, it supports the aggressive behavior of signet ring cell mixed histology in EGC. Therefore, in cases of EGC with mixed histology in the tumor biopsy and endoscopically resected specimen, we should explain the potentially aggressive behavior of the cancer to the patient, and should provide the opportunity for additional surgical resection.
This case was within the guideline indications, although it contained a partly signet ring cell component, but it recurred after 19 months with perigastric lymph node and liver metastases in spite of regular close follow-up. This case shows that additional surgical resection after ESD, even though within the guideline indications, should be recommended in mild or moderately differentiated EGC with a mixed histology, especially a signet ring cell component.
Go to : 
References
1. Gotoda T, Jung HY. Endoscopic resection (endoscopic mucosal resection/ endoscopic submucosal dissection) for early gastric cancer. Dig Endosc. 2013; 25(Suppl 1):55–63. PMID: 23362925.

2. Coda S, Lee SY, Gotoda T. Endoscopic mucosal resection and endoscopic submucosal dissection as treatments for early gastrointestinal cancers in Western countries. Gut Liver. 2007; 1:12–21. PMID: 20485653.

3. Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007; 10:1–11. PMID: 17334711.

4. Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000; 3:219–225. PMID: 11984739.

5. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma: 2nd English edition. Gastric Cancer. 1998; 1:10–24. PMID: 11957040.
6. Iwamoto J, Mizokami Y, Ito M, et al. Clinicopathological features of undifferentiated mixed type early gastric cancer treated with endoscopic submucosal dissection. Hepatogastroenterology. 2010; 57:185–190. PMID: 20422899.
7. Shimizu H, Ichikawa D, Komatsu S, et al. The decision criterion of histological mixed type in "T1/T2" gastric carcinoma: comparison between TNM classification and Japanese Classification of Gastric Cancer. J Surg Oncol. 2012; 105:800–804. PMID: 22189799.
8. Choi MH, Hong SJ, Han JP, et al. Therapeutic outcomes of endoscopic submucosal dissection in undifferentiated-type early gastric cancer. Korean J Gastroenterol. 2013; 61:196–202. PMID: 23624733.

9. Terada T. Primary signet-ring cell carcinoma of the pancreas diagnosed by endoscopic retrograde pancreatic duct biopsy: a case report with an immunohistochemical study. Endoscopy. 2012; 44(Suppl 2 UCTN):E141–E142. PMID: 22619039.

10. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123. PMID: 21573742.
11. Huh CW, Jung da H, Kim JH, et al. Signet ring cell mixed histology may show more aggressive behavior than other histologies in early gastric cancer. J Surg Oncol. 2013; 107:124–129. PMID: 22991272.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download