Abstract
Barrett esophagus is recognized as a risk factor for the development of dysplasia and adenocarcinoma of the esophagus. Cancer is usually diagnosed at an advanced stage with a 5-year survival rate of 15%. Most of these patients present de novo and are not part of a surveillance program. Endoscopic screening with improvement in recognition of early lesions may change this pattern. In the past, patients diagnosed with dysplasia and mucosal cancer were best managed by esophagectomy. Endoscopic techniques such as endoscopic mucosal resection and radiofrequency ablation have resulted in high curative rates and a shift away from esophagectomy. This pathway is supported by the literature review of esophagectomies performed for mucosal disease, as well as pathologists' interpretation of endoscopic mucosal specimens, which document the low risk of lymph node metastasis. The role of endoscopic therapy for superficial submucosal disease continues to be a challenge.
In the United States, the burden of esophageal cancer in 2007 was 16,640 new cases and 14,500 deaths with a 5-year survival rate of only 15%.1 Of these cases, 65% were adenocarcinoma associated with Barrett esophagus (BE). This compares with a 5-year survival rate of 60% for patients diagnosed with colorectal cancer; although colorectal cancer is nine times more common, it is four times less lethal.
Although BE is relatively common, only a small percentage of patients develop dysplasia and esophageal adenocarcinoma (EAC).2,3 There is a gradual evolution from low-grade dysplasia (LGD) to high-grade dysplasia (HGD), intramucosal cancer (IMCa), and lethal disease; this evolution takes many years.4
The risk of developing EAC among patients with BE is 0.12% to 0.31% per year.3,5 The most important risk factor seems to be the presence of intestinal metaplasia (IM) rather than columnar mucosa (0.31% vs. 0.06%).5
The rate of progression of LGD is unclear. However, recent studies suggest that it is more significant than previously estimated.6 In patients with HGD, the progression to EAC is 6% a year.7
Although there is no question that BE is premalignant and that EAC is usually first diagnosed at an advanced and lethal stage, a cost-effective surveillance strategy has not been established.8 This is related to the fact that only a small proportion of patients with BE develop dysplasia, and currently, there is no selection factor short of endoscopy for every patient who has reflux.
The endoscopic algorithm for treating dysplasia involves several factors such as its length (short or long segment), circumferential component, the histology of the biopsy (LGD, HGD, or IMCa), presence of a visible lesion and its size, and whether there is submucosal invasion. The extent of BE can be further defined using the Prague classification.9
Endoscopic therapy with either endoscopic mucosal resection (EMR) or radiofrequency ablation (RFA) should be used only when risk of lymph node metastasis (LNM) is negligible and a cure is expected after local treatment.
The risk of LNM is related to the invasion depth, histological lesion type, and lymphatic or vascular involvement.
The obligation of the endoscopist is to ensure that the patient is not understaged and does not lose the chance for cure. In the late 1990s and early 2000s, there was controversy between the endoscopic treatment of mucosal dysplasia and the role of esophagectomy. This was related to the misinterpretation of the term adenocarcinoma as to its depth in the mucosa and subsequent risk of LNM. Fortunately, with the review of thousands of esophagectomy specimens, better understanding by pathologists of the structure of Barrett mucosa, and the widespread use of EMR, the risk of endoscopic therapy was significantly downgraded, and esophagectomy is now seldom recommended for well-staged mucosal disease.10
The safety and appropriateness of endoscopic therapy has been supported by numerous meta-analyses of esophagectomy specimens and their lymph node (LN) statuses. In a systematic review including 70 studies and 1,874 patients, Dunbar and Spechler11 reported a 0% risk for LNM among 524 patients with HGD and 1.93% among 1,350 patients with IMCa. A landmark study from the Cleveland Clinic that specifically addressed the significance of the duplicated muscularis mucosae in esophagectomy specimens demonstrated that only 1/150 patients (0.7%) who underwent esophagectomy for mucosal disease had LNM.12 In retrospect, using current endoscopic devices, 149 of these patients could have been spared an esophagectomy and had their esophagus preserved. In the same study, 3/35 patients (8.3%) with superficial submucosal disease (sm1) had LNM.
The decision and results of endoscopic therapy must always be balanced with the operative mortality of esophagectomy. Given that esophagectomy has a mortality rate of approximately 2% in expert centers and a low risk of LNM, esophagectomy is not justified for most cases of IMCa. One review reported a mortality rate of 1.2% in the surgical group compared with 0.04% in the endoscopic group for similarly staged patients.13
Pech et al.14 showed a 5-year overall survival rate of 91% for IMCa and an estimated 12-year survival rate of 75% in 1,000 patients after endoscopic treatment of IMCa. The median follow-up period was 57 months. Complications were experienced in 1.5% of patients and managed conservatively. Just two patients (0.2%) died from BE-related cancer.14
Ngamruengphong et al.15 found similar long-term survival rates for patients treated for IMCa either with esophagectomy or endoscopic therapy. Zehetner et al.16 compared endoscopic resection and ablation to esophagectomy for the treatment of HGD and IMCa and reported similar results with less morbidity in the endoscopy group as well as similar short-term survival rates compared with esophagectomy.
Although the pendulum is shifting away from esophagectomy to endoscopic therapy, esophagectomy continues to play a significant role in a subset of patients with high-risk mucosal disease as well as those with submucosal involvement. The management of superficial submucosal disease is described in detail in a later section.
Endoscopic modalities include EMR and RFA either separately or more often in sequential combination. EMR supplies the pathologist with a large sample of the mucosa and part of the submucosa. This allows staging and simultaneous eradication. Ablation techniques have evolved from photodynamic therapy (PDT) to argon plasma coagulation (APC) and now devices that deliver radiofrequency energy. These techniques will be discussed in separate segments below.
Staging determines treatment. Endoscopic resection with either EMR or endoscopic submucosal dissection (ESD) is the most accurate method for pathological diagnosis and staging.17,18 Specimens, when retrieved, should be pinned with the deep surface down on stiff material.
For flat nonnodular BE, conventional biopsies by the Seattle protocol should be sufficient to commence endoscopic therapy.19 Nodular or recognizable lesions should always be resected (Fig. 1).
There is very good correlation between preoperative EMR staging compared with the postoperative T staging of esophagectomy specimens.20 The benefit of EMR in staging has been demonstrated by the fact that in >25% of patients there was either upgrading or downgrading compared with their previous biopsy specimens.21,22 In a multicenter study, Wani et al.23 showed an overall change in 31% of patients; 11% were upgraded and 20% downgraded from their initial pathology interpretation. Similar results have been reported for ESD.24
The role of routine endoscopic ultrasound (EUS) is controversial because of its lack of accurate staging of mucosal or submucosal disease (T1a or T1b) with significant understaging (15% to 25%) or overstaging (5% to 15%) compared with EMR.21,25 EUS is more accurate in regional LN staging (N) compared with either computed tomography or positron emission tomography, but these radiological tests are still performed in practice despite a lack of good clinical evidence as to their usefulness and accuracy.25-27 They are routinely used to stage for remote disease in patients who have been assessed for esophagectomy.
In the case of nodular lesions that feel firm on probing or do not lift with injection, EUS has an obvious role in staging.
When EUS suggests abnormal LNs (≥10 mm, round in shape, smooth borders, and a hypoechoic pattern), fine needle aspiration should be considered. However, discretion should be used in passing the needle through the wall, which could be involved with dysplastic tissue.
The staging of patients with sm1 disease and the algorithm for treatment remain controversial.
The aim of endoscopic treatment is not only the cure of dysplasia but the total eradication of BE. Endoscopic therapy in conjunction with acid suppression aims at the replacement of Barrett mucosa by its native squamous epithelium. Although this regeneration occurs in most patients, it is not universal for reasons not well understood.
Endoscopic modalities include resection techniques (EMR/ESD) or ablation techniques (thermal/nonthermal), which can be used separately or combined at different sessions.
Endoscopic resection such as EMR or ESD has revolutionized the staging and eradication of BE. These techniques allow the removal of large specimens that include mucosa and submucosa suitable for predictive staging.
The two commercially available devices for piecemeal resection are cap-snare with injection and multiband ligation entrapment. The techniques have a similar success rate and their selection is related to operator preferences.28-30 Good technique for either device demands side by side resections without residual islands between individual resections (Fig. 2). Nodular areas should be marked with a cautery device to ensure wide margins. The number of resections performed at one sitting depends on the length of the recognizable, irregular mucosa. Although there is no limit in the number of resections, most endoscopists will not exceed 50% to 60% of the circumference. Circumferential resections for short segment disease in a single setting, although tempting, are seldom performed because of the high incidence of strictures and the availability of focal RFA.
Different studies have shown a clear benefit for shorter segments (up to 4 to 5 cm) or noncircumferential BE treated with stepwise EMR.31-34 EMR is repeated until all visible BE has been removed. Chennat et al.32 achieved a 97% complete eradication rate of IM in a group of 46 patients with noncircumferential BE and a total of 106 procedures. They reported no severe complications but a 37% stricture rate.32 Similar results were observed by Pouw et al.34 in 169 patients with BE <5 cm with a 95% neoplasia eradication rate and an 80% IM eradication rate after 32 months of follow-up. The rate of symptomatic stenosis was 50%.34 Pech et al.14 published a single-center study of 1,000 patients with IMCa and a mean 2.7 endoscopic resection procedures (91% of them with multiband ligation) per patient and a median follow-up period of 57 months. The neoplasia eradication rate was 96.3%.14 Dilation was required in only 1.3% of patients. Significant bleeding occurred in 1.4% and perforation in 0.1%.
Complications associated with piecemeal EMR are perforation, bleeding, and stenosis. The perforation rate has been surprisingly low (0% to 1%).14,31-34 Most of these perforations have been managed conservatively. In our experience with >8,000 single specimens over a 10-year period, we have not had a perforation. Bleeding during the procedure is common.28,29,35 It usually stops spontaneously with compression or with the use of coagulating forceps. Rarely have we admitted a patient because of intraprocedural bleeding. Delayed bleeding occurs in up to 3% of patients and usually stops spontaneously by the time of diagnostic endoscopy. In our experience, no patient has required either surgery or interventional radiology. Strictures are very common and occur in 20% to 40% of patients. They are usually treated with either Savary or scope balloon dilations.31-35 Many of these patients will require several dilations before they become dysphagia-free. Preventive pharmacological therapy with either topical or systemic steroids seems promising.36-38
ESD is a technique of en bloc resection of the mucosa. A viscous liquid is injected into the submucosal space, and then a resection of the mucosa and part of the submucosa is performed with specially designed dissecting knives. Pioneered in Japan mainly to treat early gastric cancers, ESD was subsequently applied to the rest of the gastrointestinal tract including the esophagus. Because of the required skill set and associated clinical issues such as inflammatory changes and fibrosis in the mucosa and submucosa, Western endoscopists have felt that dissection is problematic and hazardous in their hands.
Deprez et al.39 compared ESD with cap-snare EMR in two groups of 25 patients each with a mean length of C2M5. Eradication of dysplasia and IM in both groups was similar at 100% and 84%, respectively. ESD required more time per procedure and had a higher stenosis rate (44% vs. 20%). The cost in the EMR group was almost half the cost of ESD. One perforation in the EMR group and 2 in the ESD group appeared. Despite the fact that BE with dysplasia is many times more common in the West, only two reports on ESD have been published compared with many on EMR and ablation. This suggests that western endoscopists are not convinced of its advantage and are unwilling to learn.
Ablation therapy involves the destruction of wide areas of the esophageal mucosa without additional tissue extraction. The destruction may be thermal (APC, laser, RFA) or nonthermal. Although APC and laser have been used in the remote pass, they have now been supplanted by RFA. Nonthermal methods include PDT and cryotherapy. PDT was popular and widely used in centers of endoscopic excellence in the mid-1990s to the first decade of 2000. Although effective, because of issues related to photosensitivity, high stricture rates, and cost and then the arrival of RFA technology, PDT has virtually disappeared as a therapeutic option.
RFA uses bipolar energy controlled delivery systems to treat dysplastic (12 to 15 Joules/cm2) or nondysplastic (10 Joules/cm2) BE. RFA facilitates the destruction of wide areas in a relatively short treatment time. The depth of destruction of approximately 1,000 microns is sufficient to ablate nonnodular Barrett dysplasia. Nondysplastic Barrett epithelium is usually not more than 600 microns thick. Destruction of dysplasia can be achieved without the high risk of stenosis because the muscularis mucosae or submucosa remains intact in most instances.
The HALO system (BÂRRX Medical Covidien Inc., Sunnyvale, CA, USA) is available in several formats. Cylindrical balloons (HALO 360) are ideal for circumferential and long segments of BE (Fig. 3). Focal ablation devices (HALO 90 and HALO 60) of different lengths that fit over the endoscope tip can be used for short circumferential segments, tongues, or residual islands (Fig. 4). Recently, a through the scope ablation device (HALO-TTS) has been developed to complement the other focal devices and is easier and quicker to use.
In the first randomized, multicenter, RFA versus sham-controlled trial, Shaheen et al.40 showed a 77% versus 2.3% rate of IM eradication, 81% versus 19% rate of HGD eradication, 90% versus 23% rate for any dysplasia eradication, 2.4% versus 19% rate of progression to cancer, and 6% stricture rate over 12 months. The same investigators recently reviewed the long-term results with rates of dysplasia and IM eradication of 98% and 91%, respectively, in long-segment BE (mean length, 5 cm) after 3 years of follow-up.41
Dulai et al.42 showed no recurrence of dysplasia after 3 years but an IM recurrence rate of 35% in ultralong-segment BE (mean length, 10.8 cm) and 18% in long-segment BE (mean length, 4.7 cm). The aforementioned clinical trials demonstrate that RFA maintains its long-term effectiveness in the eradication of dysplasia and confirms its role as a pillar in endoscopic eradication.
A recent Markov analysis by Hur et al.43 suggests that RFA is cost effective in preventing the progression of HGD to cancer compared to surveillance. They suggest a role for ablation in confirmed, stable, and multifocal LGD. The role for ablation in nondysplastic BE was unclear.43
Although RFA is effective, several treatments will be required. The concern about the risk of buried glands and subsquamous cancer seems to have been exaggerated, representing <1% of patients.44-46 A similar rate of buried glands was observed in one study using 3-dimensional optical coherence tomography.47
RFA has a remarkable long-term safety profile considering the many thousands of patients treated around the world. The commonest serious complication is stricturing with rates between 6% and 14%.40-42,48 These strictures tend not to be as fibrotic or difficult to manage compared with those post-EMR. Chest pain is quite common after ablation of long segments and will usually resolve within 3 to 5 days. Mucosal lacerations with the balloon device are relatively common and yet, surprisingly, perforations seem not to occur.40-42,48
PDT involves a photochemical reaction between a photosensitizing drug activated by a specific wavelength of laser light delivered endoscopically. The interaction of drug and laser light produces cytotoxic free radicals that destroy mainly the microvasculature of the tumor leading to necrosis. The depth of necrosis is related to the concentration of the drug in the tissue and the wavelength of the light. PDT has been used since the 1970s for the cure and palliation of a wide range of cancers.
In a multicenter phase III trial, Overholt et al.49 determined the efficacy and safety of PDT with porfimer sodium (Photofirin; Pinnacle Biologic, Bannockburn, IL, USA) for HGD compared with a proton pump inhibitor showing a rate of IM eradication of 52% versus 7%, respectively, an HGD eradication rate of 77% versus 39%, and a decreased progression to cancer (13% vs. 28%) in the PDT group after 24 months of follow-up. The same investigators showed the maintenance of these results after 5 years of follow-up.50 The complications were short-lived photosensitivity and a stricture rate of 36%.49
PDT with aminolevulinic acid has a better safety and efficacy profile than PDT with porfimer sodium in patients with BE <6 cm.51
With the introduction of RFA and its excellent results in mucosal disease, PDT has virtually disappeared from the scene. In a comparative study of PDT versus RFA, Ertan et al.52 reported better results with RFA with less complications and cost for treatment of BE-related dysplasia. PDT may have a role in inoperable patients with long-segment nodular BE dysplasia. Perhaps in the future, more effective and patient-friendly photosensitizers will be developed.
Cryotherapy is a new ablative technique that freezes the mucosa at subzero temperatures using either compressed liquid nitrogen or carbon dioxide delivered with specially designed catheters. The technique produces tissue necrosis caused by the formation of extracellular and intracellular ice crystals. The depth of destruction depends on the duration of the freezing and thawing cycles. Cryotherapy facilitates the treatment of wide swaths of esophageal mucosa. Although technically attractive with a short learning curve, cryotherapy requires many sessions. Despite the fact that there have been no randomized controlled studies, Gosain et al.53 report promising results, especially with liquid nitrogen cryotherapy.
The availability of RFA has resulted in improved long-term results in patients with HGD and IMCa. Originally, the main endoscopic approach was mucosal resection followed by ablation with PDT or APC. The literature and consensus statements support a dual approach where nodular lesions are resected for cure and staging and then the resulting nonnodular mucosa is ablated with RFA.7,54,55
In a cohort study by Phoa et al.,45 among patients who underwent RFA (72% with previous EMR) for neoplastic BE, 90% remained in remission at the 5-year follow-up. All recurrences were managed endoscopically. The authors concluded that this approach is therefore an effective and durable alternative to esophagectomy.45
In their recent multicenter study of 592 patients, Gupta et al.56 showed a 33% recurrence rate of IM after previously confirmed eradication (two consecutive negative endoscopies) over 2 years. Of these recurrences, 22% were dysplastic; all had repeat endoscopic therapy and only one underwent esophagectomy. Fifty-five percent of patients had a prior EMR before RFA.56
Haidry et al.57 reported similar results in a multicenter UK study with 335 patients (72% HGD, 24% IMCa, and 4% LGD) and a mean length of 5.8 cm. Eighty-six percent of patients were free of HGD and 81% of all dysplasia. Sixty-two percent were free of IM after 12 months with a mean of 2.5 RFA procedures (done at 3-month intervals).57 Complete reversal of dysplasia was less likely for every centimeter increment in BE length. Invasive cancer developed in 10 patients (3%) at 12 months. Dysplasia progressed in 17 patients (5.1%) after 19 months. Symptomatic strictures developed in 9% of patients and were treated by endoscopic dilatation. For 19 months after therapy started, 94% of patients remained with no dysplasia recurrence.
Traditionally, these procedures are performed at different times, but in selected patients, the two procedures (EMR followed by RFA) could be performed simultaneously. However, the postprocedure discomfort of odynophagia and dysphagia may be more intense.
The usefulness of ESD followed by RFA was demonstrated in the IM of 30 patients with HGD/IMCa and visible lesions >3 cm. Complete resection of the targeted area was achieved in 97% (90% en bloc resection). Minor delayed bleeding occurred in 7% of patients. Complete resection was histologically confirmed (R0 resection) in only 38% of the 26 patients with HGD or IMCa. After a median follow-up period of 17 months, complete remission of neoplasia was 97%. However, experts at this endoscopic center concluded that although ESD is feasible and safe, it does not achieve sufficient R0 resection rates to warrant its recommendation over piecemeal EMR.24
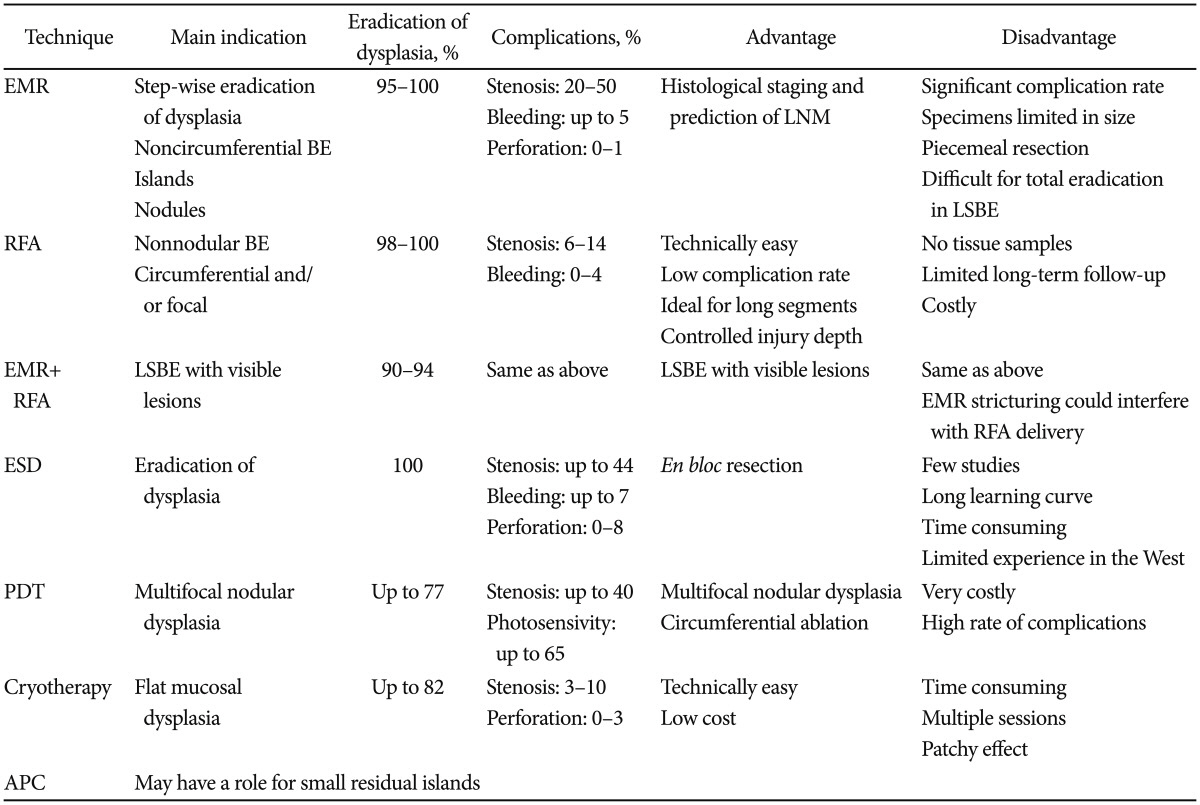
We summarize different treatment modalities in Table 1.
A special subset includes patients with portal hypertension, possible varices, low platelets, possible coagulopathy, and BE with dysplasia. In these special circumstances, if varices are not seen, the esophagus should be examined with EUS-Doppler to determine whether there are submucosal varices. If varices are recognized, before embarking on treatment of the dysplasia, the varices should be eradicated with either standard rubber banding or injection therapy. One month later, the esophagus is reassessed with EUS, and if the eradication is confirmed, treatment with the multiband ligation EMR system or possible ablation with RFA should be considered.
If concern persists about the risk of bleeding, multiple rubber-band ligations can be applied without snare excisions. Biopsies can be safely performed from the apex of the band-entrapped pseudopolyp.58,59 An indicator of a good response would be the replacement of the Barrett mucosa by squamous reepithelialization. This process can be repeated every 3 to 4 weeks until all of the dysplastic tissue has been removed.
The current guidelines by professional societies state that although patients with LGD should be followed independently more than nondysplastic patients, endoscopic treatment has not been recommended because of the lack of long-term, randomized, and controlled trials showing efficacy.7,54 The concept that LGD should be placed in the category of observational surveillance is supported by Bhat et al.5 and Wani et al.60 who reported progression rates to HGD or IMCa <1.4% a year. However, Curvers et al.6 documented a 13.4% progression rate to HGD or IMCa for true LGD (confirmed by at least two expert GI pathologists). They comment that histological interpretation is crucial and that in their experience patients with documented histological LGD have an increased risk of progression and should be treated.6 It is also our clinical recommendation that patients with consistent and persistent multifocal LGD should undergo endoscopic therapy. This would include patients aged <50 years and those with a family history of BE-related cancer.
Traditionally, any involvement of the submucosa was felt to have a high risk of LNM and, therefore, in the operable patient, was an indication for esophagectomy. The management of patients with sm1 (<500 µm into the submucosa) is controversial.
Generally, the literature does not support endoscopic treatment because of LNM rates of at least 8% for sm1 and 15% for sm2 and sm3.12,61-63
A review of esophagectomy specimens from the Mayo Clinic and Cleveland Clinic offers important insight into LN involvement in sm1 patients from 8% to 12%.12,61 These studies come from high-volume expert surgical centers with operative mortality rates in the range of 2%.
Manner et al.64 report good and safe results for endoscopic treatment of submucosal adenocarcinomas in low-risk lesions: macroscopically polypoid or flat, involving one-third or less of the submucosal thickness (sm1), good-moderate grade of differentiation (G1/2), and no invasion into the lymph vessels or veins. The complete endoluminal remission was higher in focal neoplasms <2 cm (97% vs. 77%). Metachronous neoplasms were observed in 19% of patients undergoing an endoscopic resection. One patient developed an LNM (1.9%). No tumor-associated deaths were observed, and the estimated 5-year survival rate was 84%. There was a 1.5% rate for major endoscopic complications with no procedure-related deaths. Similar results for risk of LNM were reported by Alvarez Herrero et al.65
The obvious dilemma and the challenge for the future will be to have some histological or imaging methods to better define the absence of LN involvement with high negative predictive value that matches the operative mortality and could eliminate the need for esophagectomy in 90% of sm1 patients.
Patients treated endoscopically require close and long-term follow-up with endoscopic and EUS surveillance every 3 months for 2 years and then every 6 months for at least 5 years.
The aim of treatment of BE with dysplasia is: 1) to cure the dysplasia and prevent progression and 2) to ensure that all of the BE is eradicated to minimize the risk of metachronous lesions. This requires an endoscopic center that has a robust database and appropriate personnel that follow patients on a regular basis with endoscopy and other related imaging modalities.
Recurrence of advanced dysplasia (HGD or IMCa), although not common, has been found in more than 15% of patients.14,28,29,64,66 This is a crucial point in the ongoing surveillance of these patients, as many can be retreated to achieve permanent cure.
After BE eradication, detailed review of all mucosa using different imaging modalities will need to be conducted in different sessions. A first review is recommended after 3 months, the next after 6 months, the following after 6 months, and if no recurrence is found then yearly depending on the histological findings.
van Vilsteren et al.67 reported that active reflux esophagitis, failure of reepithelialization after previous EMR, esophageal stricturing, and years of neoplasia pre-RFA predicted a poor initial response after circumferential RFA. The importance of reflux as a poor predictor of response is further supported by Krishnan et al.68 Other poor indicators are the length of BE, the size of the nodule, and the degree of differentiation.14 Stricturing as a result of either EMR or RFA may interfere with technical success to endoscopic eradication.
Although Shaheen et al.69 did not show better results for BE eradication with RFA when antireflux surgery was performed before endoscopic therapy, a subset of patients with recurrent high-volume reflux, persisting esophagitis, and failed eradication might benefit from antireflux surgery.
Esophagectomy has been the traditional therapy for HGD and IMCa. This evolved through a misinterpretation of the risk of LNM at a time when there were no good endoscopic options. Several meta-analyses have since supported the nonsurgical approach for HGD and IMCa with degrees of LN involvement at least less than reported operative mortality.7,12,16 The introduction of endoscopic resection revolutionized the staging of mucosal disease and allowed for the down staging of the LNM risk, as interpreted by expert gastrointestinal pathologists. The addition of mucosal ablation techniques to endoscopic resection has resulted in outcomes equal to esophagectomy. Esophagectomy may have a role in patients with poorly differentiated IMCa as well as mucosal lymphovascular invasion or for difficult to ablate mucosal lesions in young, operable patients. The role of esophagectomy in patients with well-staged sm1 disease is controversial. This shift of the pendulum away from esophagectomy to endoscopic cure is now recommended even in surgical units that in the past were skeptical of the endoscopic approach.16
The management of dysplasia in BE has been revolutionized because of the widespread introduction of endoscopic-directed EMR and RFA as well as a better understanding of the disease pathology. The pendulum has swung away from esophagectomy to endoscopic modalities for long-term cure of HGD and IMCa.
Esophagectomy for mucosal disease remains an option in only a small subset of operable patients.
A challenge will be the accurate definition of LN risk so that patients with sm1 or even sm2 may be better staged and selected and endoscopic therapy can be an option for cure.
The future may bring more accurate targeting for the diagnosis of occult mucosal dysplasia with yet to be developed biomarkers and monoclonal antibodies. Some of these antibodies may incorporate cancer destroying drugs and/or photosensitive agents. A new era in endoscopic-directed diagnosis and therapy could emerge.
Patients with BE should be managed and followed long term in expert centers with a dedicated team of physicians, nurses, and research personnel.
References
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010; 60:277–300. PMID: 20610543.

2. Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013; 19:5598–5606. PMID: 24039351.

3. Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011; 365:1375–1383. PMID: 21995385.

4. Schnell TG, Sontag SJ, Chejfec G, et al. Long-term nonsurgical management of Barrett's esophagus with high-grade dysplasia. Gastroenterology. 2001; 120:1607–1619. PMID: 11375943.

5. Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011; 103:1049–1057. PMID: 21680910.

6. Curvers WL, ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett's esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010; 105:1523–1530. PMID: 20461069.

7. Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association. American Gastroenterological Association technical review on the management of Barrett's esophagus. Gastroenterology. 2011; 140:e18–e52. PMID: 21376939.

8. Gordon LG, Mayne GC, Hirst NG, et al. Cost-effectiveness of endoscopic surveillance of non-dysplastic Barrett's esophagus. Gastrointest Endosc. Epub 2013 Sep 27. DOI: 10.1016/j.gie.2013.07.046.

9. Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology. 2006; 131:1392–1399. PMID: 17101315.
10. Vieth M, Ell C, Gossner L, May A, Stolte M. Histological analysis of endoscopic resection specimens from 326 patients with Barrett's esophagus and early neoplasia. Endoscopy. 2004; 36:776–781. PMID: 15326572.

11. Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett's esophagus: a systematic review. Am J Gastroenterol. 2012; 107:850–862. PMID: 22488081.

12. Kaneshiro DK, Post JC, Rybicki L, Rice TW, Goldblum JR. Clinical significance of the duplicated muscularis mucosae in Barrett esophagus-related superficial adenocarcinoma. Am J Surg Pathol. 2011; 35:697–700. PMID: 21490446.

13. Menon D, Stafinski T, Wu H, Lau D, Wong C. Endoscopic treatments for Barrett's esophagus: a systematic review of safety and effectiveness compared to esophagectomy. BMC Gastroenterol. 2010; 10:111. PMID: 20875123.

14. Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. Epub 2013 Nov 20. DOI: 10.1053/j.gastro.2013.11.006.

15. Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol. 2013; 11:1424–1429. PMID: 23735443.

16. Zehetner J, DeMeester SR, Hagen JA, et al. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg. 2011; 141:39–47. PMID: 21055772.

17. Moss A, Bourke MJ, Hourigan LF, et al. Endoscopic resection for Barrett's high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010; 105:1276–1283. PMID: 20179694.

18. ASGE Technology Committee. Kantsevoy SV, Adler DG, et al. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008; 68:11–18. PMID: 18577472.

19. Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, Reid BJ. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett's esophagus. Gastroenterology. 1993; 105:40–50. PMID: 8514061.

20. Namasivayam V, Wang KK, Prasad GA. Endoscopic mucosal resection in the management of esophageal neoplasia: current status and future directions. Clin Gastroenterol Hepatol. 2010; 8:743–754. PMID: 20541628.

21. Larghi A, Lightdale CJ, Memeo L, Bhagat G, Okpara N, Rotterdam H. EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett's esophagus. Gastrointest Endosc. 2005; 62:16–23. PMID: 15990814.

22. Bhat YM, Furth EE, Brensinger CM, Ginsberg GG. Endoscopic resection with ligation using a multi-band mucosectomy system in Barrett's esophagus with high-grade dysplasia and intramucosal carcinoma. Therap Adv Gastroenterol. 2009; 2:323–330.

23. Wani S, Abrams J, Edmundowicz SA, et al. Endoscopic mucosal resection results in change of histologic diagnosis in Barrett's esophagus patients with visible and flat neoplasia: a multicenter cohort study. Dig Dis Sci. 2013; 58:1703–1709. PMID: 23633158.

24. Neuhaus H, Terheggen G, Rutz EM, Vieth M, Schumacher B. Endoscopic submucosal dissection plus radiofrequency ablation of neoplastic Barrett's esophagus. Endoscopy. 2012; 44:1105–1113. PMID: 22968641.

25. Pech O, May A, Günter E, Gossner L, Ell C. The impact of endoscopic ultrasound and computed tomography on the TNM staging of early cancer in Barrett's esophagus. Am J Gastroenterol. 2006; 101:2223–2229. PMID: 17032186.

26. Buskens CJ, Westerterp M, Lagarde SM, Bergman JJ, ten Kate FJ, van Lanschot JJ. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc. 2004; 60:703–710. PMID: 15557945.

27. Keswani RN, Early DS, Edmundowicz SA, et al. Routine positron emission tomography does not alter nodal staging in patients undergoing EUS-guided FNA for esophageal cancer. Gastrointest Endosc. 2009; 69:1210–1217. PMID: 19012886.

28. Conio M, Repici A, Cestari R, et al. Endoscopic mucosal resection for high-grade dysplasia and intramucosal carcinoma in Barrett's esophagus: an Italian experience. World J Gastroenterol. 2005; 11:6650–6655. PMID: 16425359.

29. Lopes CV, Hela M, Pesenti C, et al. Circumferential endoscopic resection of Barrett's esophagus with high-grade dysplasia or early adenocarcinoma. Surg Endosc. 2007; 21:820–824. PMID: 17294308.

30. Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett's esophagus. Gastrointest Endosc. 2000; 52:328–332. PMID: 10968845.

31. Chung A, Bourke MJ, Hourigan LF, et al. Complete Barrett's excision by stepwise endoscopic resection in short-segment disease: long term outcomes and predictors of stricture. Endoscopy. 2011; 43:1025–1032. PMID: 22068701.

32. Chennat J, Konda VJ, Ross AS, et al. Complete Barrett's eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma: an American single-center experience. Am J Gastroenterol. 2009; 104:2684–2692. PMID: 19690526.
33. Peters FP, Krishnadath KK, Rygiel AM, et al. Stepwise radical endoscopic resection of the complete Barrett's esophagus with early neoplasia successfully eradicates pre-existing genetic abnormalities. Am J Gastroenterol. 2007; 102:1853–1861. PMID: 17509033.

34. Pouw RE, Seewald S, Gondrie JJ, et al. Stepwise radical endoscopic resection for eradication of Barrett's oesophagus with early neoplasia in a cohort of 169 patients. Gut. 2010; 59:1169–1177. PMID: 20525701.

35. Soehendra N, Seewald S, Groth S, et al. Use of modified multiband ligator facilitates circumferential EMR in Barrett's esophagus (with video). Gastrointest Endosc. 2006; 63:847–852. PMID: 16650552.

36. Sato H, Inoue H, Kobayashi Y, et al. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc. 2013; 78:250–257. PMID: 23453294.

37. Hashimoto S, Kobayashi M, Takeuchi M, Sato Y, Narisawa R, Aoyagi Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc. 2011; 74:1389–1393. PMID: 22136782.

38. Isomoto H, Yamaguchi N, Nakayama T, et al. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol. 2011; 11:46. PMID: 21542926.

39. Deprez PH, Piessevaux H, Aouattah T, Yeung RC, Sempoux C, Jouret-Mourin A. ESD in Barrett's esophagus high grade dysplasia and mucosal cancer: prospective comparison with CAP mucosectomy. Gastrointest Endosc. 2010; 71:AB126.
40. Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009; 360:2277–2288. PMID: 19474425.
41. Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology. 2011; 141:460–468. PMID: 21679712.
42. Dulai PS, Pohl H, Levenick JM, Gordon SR, MacKenzie TA, Rothstein RI. Radiofrequency ablation for long- and ultralong-segment Barrett's esophagus: a comparative long-term follow-up study. Gastrointest Endosc. 2013; 77:534–541. PMID: 23290719.

43. Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett's esophagus. Gastroenterology. 2012; 143:567–575. PMID: 22626608.

44. Gray NA, Odze RD, Spechler SJ. Buried metaplasia after endoscopic ablation of Barrett's esophagus: a systematic review. Am J Gastroenterol. 2011; 106:1899–1908. PMID: 21826111.

45. Phoa KN, Pouw RE, van Vilsteren FG, et al. Remission of Barrett's esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology. 2013; 145:96–104. PMID: 23542068.

46. Yuan J, Hernandez JC, Ratuapli SK, et al. Prevalence of buried Barrett's metaplasia in patients before and after radiofrequency ablation. Endoscopy. 2012; 44:993–997. PMID: 23108770.

47. Zhou C, Tsai TH, Lee HC, et al. Characterization of buried glands before and after radiofrequency ablation by using 3-dimensional optical coherence tomography (with videos). Gastrointest Endosc. 2012; 76:32–40. PMID: 22482920.

48. Bulsiewicz WJ, Kim HP, Dellon ES, et al. Safety and efficacy of endoscopic mucosal therapy with radiofrequency ablation for patients with neoplastic Barrett's esophagus. Clin Gastroenterol Hepatol. 2013; 11:636–642. PMID: 23103824.

49. Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005; 62:488–498. PMID: 16185958.

50. Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc. 2007; 66:460–468. PMID: 17643436.

51. Dunn JM, Mackenzie GD, Banks MR, et al. A randomised controlled trial of ALA vs. Photofrin photodynamic therapy for high-grade dysplasia arising in Barrett's oesophagus. Lasers Med Sci. 2013; 28:707–715. PMID: 22699800.

52. Ertan A, Zaheer I, Correa AM, Thosani N, Blackmon SH. Photodynamic therapy vs radiofrequency ablation for Barrett's dysplasia: efficacy, safety and cost-comparison. World J Gastroenterol. 2013; 19:7106–7113. PMID: 24222954.
53. Gosain S, Mercer K, Twaddell WS, Uradomo L, Greenwald BD. Liquid nitrogen spray cryotherapy in Barrett's esophagus with high-grade dysplasia: long-term results. Gastrointest Endosc. 2013; 78:260–265. PMID: 23622979.

54. Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014; 63:7–42. PMID: 24165758.

55. Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett's dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology. 2012; 143:336–346. PMID: 22537613.
56. Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett's esophagus: results from a US Multicenter Consortium. Gastroenterology. 2013; 145:79–86. PMID: 23499759.

57. Haidry RJ, Dunn JM, Butt MA, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic barrett's esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology. 2013; 145:87–95. PMID: 23542069.

58. Diaz-Cervantes E, De-la-Torre-Bravo A, Spechler SJ, et al. Banding without resection (endoscopic mucosal ligation) as a novel approach for the ablation of short-segment Barrett's epithelium: results of a pilot study. Am J Gastroenterol. 2007; 102:1640–1645. PMID: 17488252.

59. Raftopoulos SC, Efthymiou M, May G, Marcon N. Dysplastic Barrett's esophagus in cirrhosis: a treatment dilemma. Am J Gastroenterol. 2011; 106:1724–1726. PMID: 21897417.

60. Wani S, Falk GW, Post J, et al. Risk factors for progression of low-grade dysplasia in patients with Barrett's esophagus. Gastroenterology. 2011; 141:1179–1186. PMID: 21723218.

61. Badreddine RJ, Prasad GA, Lewis JT, et al. Depth of submucosal invasion does not predict lymph node metastasis and survival of patients with esophageal carcinoma. Clin Gastroenterol Hepatol. 2010; 8:248–253. PMID: 19948247.

62. Gockel I, Sgourakis G, Lyros O, et al. Risk of lymph node metastasis in submucosal esophageal cancer: a review of surgically resected patients. Expert Rev Gastroenterol Hepatol. 2011; 5:371–384. PMID: 21651355.

63. Nentwich MF, von Loga K, Reeh M, et al. Depth of submucosal tumor infiltration and its relevance in lymphatic metastasis formation for T1b squamous cell and adenocarcinomas of the esophagus. J Gastrointest Surg. Epub 2013 Oct 4. DOI: 10.1007/s11605-013-2367-2.

64. Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol. 2013; 11:630–635. PMID: 23357492.

65. Alvarez Herrero L, Pouw RE, van Vilsteren FG, et al. Risk of lymph node metastasis associated with deeper invasion by early adenocarcinoma of the esophagus and cardia: study based on endoscopic resection specimens. Endoscopy. 2010; 42:1030–1036. PMID: 20960392.

66. Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut. 2008; 57:1200–1206. PMID: 18460553.

67. van Vilsteren FG, Alvarez Herrero L, Pouw RE, et al. Predictive factors for initial treatment response after circumferential radiofrequency ablation for Barrett's esophagus with early neoplasia: a prospective multicenter study. Endoscopy. 2013; 45:516–525. PMID: 23580412.

68. Krishnan K, Pandolfino JE, Kahrilas PJ, Keefer L, Boris L, Komanduri S. Increased risk for persistent intestinal metaplasia in patients with Barrett's esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology. 2012; 143:576–581. PMID: 22609385.

69. Shaheen NJ, Kim HP, Bulsiewicz WJ, et al. Prior fundoplication does not improve safety or efficacy outcomes of radiofrequency ablation: results from the U.S. RFA Registry. J Gastrointest Surg. 2013; 17:21–28. PMID: 22965650.

Fig. 1
(A) Nodular lesion (0-IIa+IIb) 1 cm above the gastroesophageal junction. The reported histology was intramucosal adenocarcinoma involving the muscularis mucosae - M3. (B) Mucosal defect after multiband resection.

Fig. 2
(A) Nodular lesion (0-IIa+IIb) 1 cm above the gastroesophageal junction. The reported histology was intramucosal adenocarcinoma involving the muscularis mucosae - M3. (B) Mucosal defect after multiband resection.
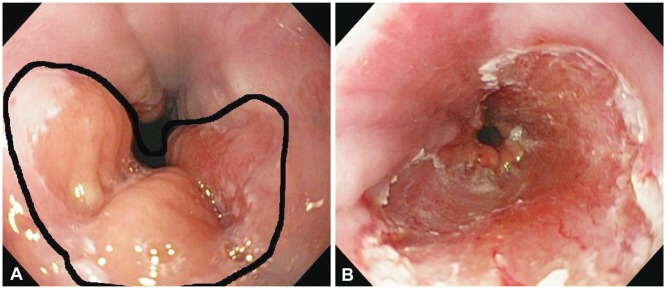
Fig. 3
(A) Nonnodular long segment of Barrett esophagus. (B) HALO 360 device immediately after deflation. (C) Mucosa immediately post-application.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download