Abstract
Patients with inflammatory bowel disease (IBD) have an increased risk of developing colorectal cancer (CRC). Accordingly, the duration and anatomic extent of the disease have been known to affect the development of IBD-related CRC. When CRC occurs in patients with IBD, unlike in sporadic CRC, it is difficult to detect the lesions because of mucosal changes caused by inflammation. In addition, the tumor types vary with ill-circumscribed lesions, and the cancer is difficult to diagnose and remedy at an early stage. For the diagnosis of CRC in patients with IBD, screening endoscopy is recommended 8 to 10 years after the IBD diagnosis, and surveillance colonoscopy is recommended every 1 to 2 years thereafter. The recent development of targeted biopsies using chromoendoscopy and relatively newer endoscopic techniques helps in the early diagnosis of CRC in patients with IBD. A total proctocolectomy is advisable when high-grade dysplasia or multifocal low-grade dysplasia is confirmed by screening endoscopy or surveillance colonoscopy or if a nonadenoma-like dysplasia-associated lesion or mass is detected. Currently, pharmacotherapies are being extensively studied as a way to prevent IBD-related CRC.
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract that includes Crohn's disease (CD) and ulcerative colitis (UC). The incidence of IBD is increasing worldwide, including Asia, where the incidence rate of IBD was previously reported to be relatively low.1 Chronic inflammation is associated with malignancy, and chronic colonic inflammation from IBD increases the risk of colorectal cancer (CRC).2,3 The incidence of CRC in patients with IBD is reportedly six times higher than that in the general population, and CRC accounts for 10% to 15% of deaths in patients with IBD.4
In patients with UC, the annual incidence rate of CRC was previously reported at 0.3%, with the cumulative rate being 1.6% at 10 years, 8.3% at 20 years, and 18.4% at 30 years.5 However, in more recent studies, the cumulative rate of CRC has decreased to 2.5% at 20 years, 7.6% at 30 years, and 10.8% at 40 years.6 Additionally, the incidence of CRC in patients with UC has decreased, with the most recent incidence rate being only two to three times higher than that in the general population.7 The Korean Association for the Study of Intestinal Diseases carried out a nationwide study in Korea and reported that the overall prevalence of CRC in patients with UC was 0.37%. The study also reported that the estimated cumulative risk of UC-associated CRC for patients who had UC was 0.7% at 10 years, 7.9% at 20 years, and 33.2% at 30 years. Unfortunately, most UC-associated CRCs were diagnosed in advanced stages. The authors concluded that the cumulative incidence of UC-associated CRCs in Korea was comparable to that in Western countries.8
The overall survival rate in IBD is not markedly lower than that in the general population. It is presumed that the factors behind decreases in the cumulative incidence rates of CRC include regular colonoscopic surveillance and improved medical therapies to reduce inflammation.
In this article, we discuss adequate timing and techniques for screening endoscopy and surveillance colonoscopy as methods to provide an early diagnosis of CRC in patients with IBD, in addition to effective biopsy methods, dysplasia treatment, and chemoprophylaxis for preventing IBD-related CRC.
Multiple risk factors for the development of CRC in patients with IBD have been identified. Persistent inflammation is believed to play a crucial and direct role in the development of CRC in patients with IBD,9 and the degree of endoscopic and histological inflammation correlates with the incidence of CRC.10 These observations support the evidence that CRC risk increases with disease duration and anatomic extent of colitis, and the risk of IBD-related CRC becomes appreciable after 7 years in patients with UC.5 The incidence of CRC in patients with extensive colitis is increased by 14.8 times. In patients with left-sided colitis, it is increased by three times, whereas in patients with proctitis, it is similar to that in the general population.11 The concomitant presence of primary sclerosing cholangitis (PSC) increases the risk of CRC in patients with UC, with the cumulative risk of CRC reported to be 25% at 10 years.12,13,14,15 Additionally, patients with IBD and a family history of CRC are suspected to have a two to five times higher incidence of developing CRC than patients without a family history.9
Compared with sporadic CRC, IBD-associated CRC has a few clinical differences. The diagnosis of CRC in patients with IBD is made at an earlier age than that in the general population, the median age of diagnosis is the fifth decade, and malignancy is more often found in the proximal colon.16 IBD-related CRC has a higher frequency of two or more synchronous CRCs, and its characteristically flat and nonpolypoid dysplasia with poor differentiation facilitates the development of diffuse cancer.17 Malignancy is difficult to identify by endoscopy in patients with UC because of the inflammation-induced changes in the colonic mucosa; tumor margins are unclear, and tumors tend to develop in variable forms. Some studies have reported no significant difference in mortality between IBD-related CRC and sporadic CRC,16,18 but most studies have shown that the mortality rate in patients with IBD-related CRC is 1.5 to 2 times higher than that in patients with sporadic CRC.19,20,21,22 Such a tendency was reportedly more notable in men diagnosed with IBD prior to age 60.20
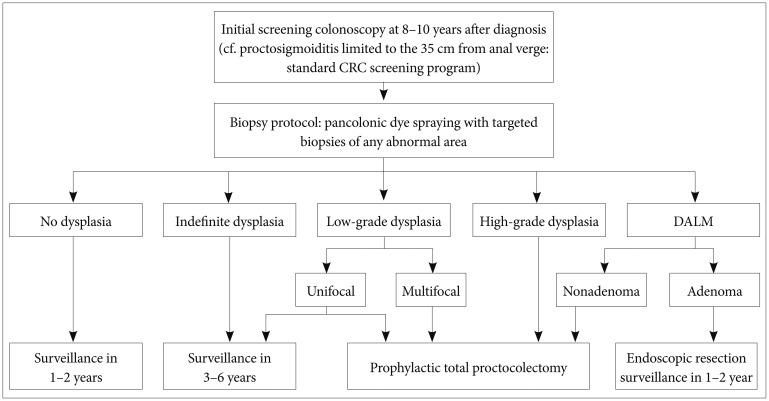
The goal of most surveillance programs is early detection of CRC and mortality reduction in patients with IBD. However, the optimal surveillance strategy in patients with IBD remains controversial. Although it is unclear whether regular colonoscopy in patients with IBD can increase survival rates, regular follow-up colonoscopies are recommended to enable early detection of malignancy.23,24 In all patients with extensive colitis and left-sided colitis, screening endoscopy is recommended 8 to 10 years after diagnosis.3,25,26,27,28,29 However, for those with proctosigmoiditis limited to within 35 cm from the anal verge, the same standard CRC screening as implemented for the general population is adequate because the incidence risk is unlikely to increase.27,30 The interval for follow-up surveillance colonoscopy depends on the presence or absence of dysplasia but should be performed at least every 1 to 2 years.3,27,30,31,32
Patients with a family history of CRC in a first-degree relative younger than 50 years are at high risk for IBD-related CRC. In a hospital-based study in Korea, 14.3% of UC patients with PSC were diagnosed with CRC and died of PSC-associated complications.33 Patients with a family history of CRC or a personal history of PSC should undergo surveillance colonoscopy every year starting at the point of diagnosis.29
In patients with CD involving at least one-third of the colon, screening endoscopy, and surveillance colonoscopy should be performed as in patients with UC because dysplasia and CRC tend to occur at a similar rate as in patients with UC.3,25,29
Because it is difficult to identify dysplasia when colonic mucosal inflammation is severe, in principle, screening and surveillance colonoscopy should be performed when IBD is in remission. However, screening and surveillance colonoscopy should not be unduly delayed if remission cannot be achieved.28,29
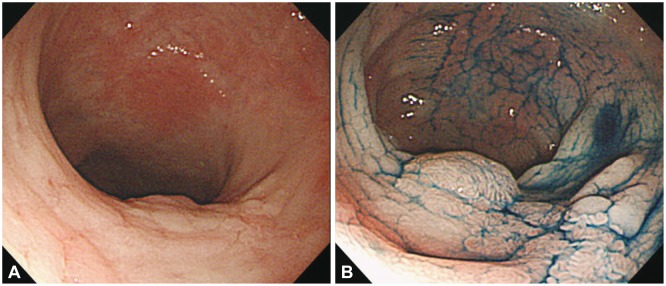
Multiple colon biopsies are often required for CRC surveillance because dysplasia in patients with IBD is flat and multifocal, making it difficult to detect grossly. As a standard method for the detection of IBD-related CRC, random 4-quadrant biopsies with regular 10-cm intervals have been recommended for at least 33 different regions of the entire colon34 in addition to biopsy of areas with mucosal irregularity.35 However, in random biopsies, less than 1% of the entire mucosal surface of the colon is sampled, leaving a very high sampling error36 and yielding a low positive rate, while also being expensive and time-consuming. Chromoendoscopy, a technique that uses dyes such as indigo carmine and methylene blue sprayed on the colonic mucosa, can provide improved visualization of fine mucosal changes when compared with conventional white light endoscopy (Fig. 1). The dysplasia detection rate is reportedly 2.0% to 8.8% in conventional white light endoscopy compared with 7.0% to 16.7% in chromoendoscopy, indicating that the latter is two to three times better at detecting dysplasia.37,38,39,40,41 Although additional time is required for the dye spraying, no time disparities occur between the two methods because of the reduced frequency of biopsies needed with chromoendoscopy relative to the random biopsy approach. The Guidelines of the British Society of Gastroenterology, updated in 2010, recommend pancolonic dye spraying with targeted biopsies of any abnormal areas.29
Ongoing studies aim to validate the effectiveness of new methods, such as confocal endomicroscopy and autofluorescence imaging. Chromoendoscopy with confocal endomicroscopy has an intraepithelial neoplasia diagnostic yield that is 2.5 times higher than that of chromoendoscopy alone and 4.75 times higher than that of conventional white light endoscopy.39,42 Data from a small study indicate that autofluorescence imaging provides a sensitivity of 87% to 100% for detecting dysplastic lesions in patients with IBD. However, possible variability between endoscopists is an issue, and these new procedures cannot be fully applied for CRC surveillance without securing validation from large-scale investigations.
Dysplasia detected by surveillance colonoscopy is categorized according to histological findings as high-grade dysplasia, low-grade dysplasia, or indefinite dysplasia.43 It is also divided into two classes based on endoscopic diagnosis: dysplasia-associated lesion or mass (DALM), which can be detected grossly, and flat dysplasia, which is not easily detected grossly but is identified by random blind biopsies.44 If high-grade dysplasia or multifocal low-grade dysplasia is confirmed histologically, a patient is at high risk of synchronous CRC and, therefore, should be referred for prophylactic total proctocolectomy.30 Patients with indefinite dysplasia are advised to reundergo surveillance colonoscopy 3 to 6 months after completing treatment for active inflammation.26 However, no consensus exists regarding the treatment of unifocal low-grade dysplasia; some studies claim that in cases where only unifocal low-grade dysplasia was found, 20% of colectomy patients had already developed cancer,45 Others suggest that merely 2% to 10% of patients develop cancer after a 10-year follow-up.46 Recently, more frequent surveillance colonoscopy was recommended for patients with unifocal low-grade dysplasia when proctocolectomy was unacceptable or not feasible.27
DALMs are often categorized as adenoma-like or nonadenoma-like. Evidence indicates that because adenoma-like DALMs tend to have a lower risk of malignancy than nonadenoma-like DALMs, they may be treated with endoscopic resection and continued regular follow-up if the lesion has been excised completely and no flat dysplasia is seen elsewhere in the colon.47 Complete proctocolectomy is proposed for nonadenoma-like DALMs because synchronous CRC has been reportedly found in up to 50% of cases (Fig. 2).48,49 With endoscopy, adenoma-like DALMs grossly appear as well-circumscribed, polypoid, and sessile lesions that do not usually accompany hemorrhage, ulceration, or necrosis, conversely, nonadenoma-like DALMs appear as irregular and ill-circumscribed lesions, usually accompanied by hemorrhage, ulceration, or necrosis.50 However, it is not always easy to distinguish between these two lesions by gross examination; therefore, resection and intense colonoscopic surveillance are advisable when no flat dysplasia is detected surrounding the lesion.51
Interest in pharmacotherapy for CRC prevention has increased because IBD-related CRC is difficult to detect early and often carries a poor prognosis even if detection is possible. Sporadic CRC passes through the adenoma-cancer pathway, whereas IBD-related CRC passes through the inflammation-dysplasia-cancer pathway.2 Accordingly, the possibility of preventing CRC via reducing chronic inflammation is suggested.
One of the first drugs studied in the chemoprevention of IBD-related CRC was 5-aminosalicylate (5-ASA), which activates the peroxisome proliferator-activated receptor-γ pathway by reducing inflammation, thereby inhibiting the excessive turnover of epithelial cells and maintaining the balance of physiological apoptosis in tumor cells.52 Furthermore, 5-ASA may reduce microsatellite instability by preventing cellular oxidative damage and interfering with subsequent DNA mutational processes in the intestinal mucosa.53 The drug is also expected to inhibit the progression to CRC by inhibiting lipoxygenase, cyclooxygenase mediators, and interleukins.54 Most relevant studies show that 5-ASA reduced the incidence of IBD-related CRC,7,55,56,57 and the effect was most pronounced when a >1.2 g/day dose was administered.58,59 In a few studies, however, 5-ASA treatment had no impact on reducing CRC occurrences,60,61 and cancer incidence even increased according to some data.10,62 Although well-designed, prospective, randomized trials are necessary, 5-ASA is now widely used to maintain remission in patients and is recommended for the chemoprevention of CRC.63,64
In patients with PSC and UC, excessive bile secretion makes it impossible for the small intestine to sufficiently absorb the bile acid, thereby irritating the proximal colon mucosa and exacerbating existing inflammation.65 Ursodeoxycholic acid (UDCA) inhibits such disproportionate bile secretion and has a preventive effect on IBD-related CRC. In one meta-analysis, subjects were divided by UDCA dose into a low-to-medium dose group with <25 mg/kg/day and a high-dose group with >25 mg/kg/day. UDCA had a preventive effect on CRC in the low-to-medium dose group, whereas the incidence of CRC increased in the high-dose group.66 Therefore, it is recommended that patients with UC and PSC take UDCA at a dose <25 mg/kg/day to prevent CRC.
Immunomodulators, such as azathioprine, 6-mercaptopurine, and methotrexate, are known to inhibit chronic inflammation and are expected to have a CRC-preventive effect in patients with IBD. Data from one group of studies indicate no significant effect of immunomodulators on IBD-related CRC incidence,10,58,67,68 but a few studies, including a recent prospective study, reported that immunomodulator use did enhance CRC prevention.55,69,70,71 It should be noted that long-term use of these drugs is reportedly associated with an increased incidence of lymphoma.72
Inflammatory cytokines, including tumor necrosis factor-α (TNF-α), may have an important role in the initiation, promotion, and progression of IBD-related CRC. TNF-α blocking agents such as infliximab and adalimumab are known to be effective for active IBD, with rapid onset of mucosal healing and maintenance of remission. Also, blocking TNF-α resulted in significantly decreased colonic neoplasm development in animal models.73 These experimental data have not yet been validated in clinical trials, but it is expected that TNF-α blocking agents can be used in chemoprevention of IBD-related CRC.
Folic acid deficiency is understood to be one cause of sporadic CRC, but its supplementation did not lead to effective prevention of IBD-related CRC.74,75 In a population-based case-control study, statin use was shown to reduce the incidence rate of CRC in both the non-IBD and IBD patient populations,76 but further studies that include IBD patients are required.
With the rapidly growing incidence of IBD, the overall occurrence of IBD-associated CRC may be growing. Screening and surveillance colonoscopy in patients with IBD is thought to enable early detection of dysplasia and cancer, making it possible to improve the prognosis of IBD-related CRC by providing patients with proactive treatments. Although controversy remains regarding adequate endoscopic intervals and methods for effective screening and surveillance, it is generally recommended that all patients with IBD receive screening endoscopy 8 to 10 years after diagnosis, followed by surveillance colonoscopy according to each patient's risk level. Efficient biopsy methods using new technologies, such as chromoendoscopy, are expected to increase the diagnosis rate of dysplasia in patients with IBD. However, what is most needed to improve the diagnosis rate of IBD-related CRC is for physicians to take the time to monitor each patient and perform thorough biopsies of the indicated areas.
References
2. Rhodes JM, Campbell BJ. Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med. 2002; 8:10–16. PMID: 11796261.

3. Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010; 138:746–774. PMID: 20141809.

4. Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011; 4:53–61. PMID: 21673876.
5. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001; 48:526–535. PMID: 11247898.

6. Rutter MD, Saunders BP, Wilkinson KH, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006; 130:1030–1038. PMID: 16618396.

7. Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005; 100:1345–1353. PMID: 15929768.

8. Kim BJ, Yang SK, Kim JS, et al. Trends of ulcerative colitis-associated colorectal cancer in Korea: A KASID study. J Gastroenterol Hepatol. 2009; 24:667–671. PMID: 19378391.

9. Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004; 287:G7–G17. PMID: 15194558.

10. Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004; 126:451–459. PMID: 14762782.

11. Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990; 323:1228–1233. PMID: 2215606.
12. Kim EY, Kim HJ, Lee CK, et al. A case of cholangiocarcinoma and colorectal cancer diagnosed simultaneously in a patient with ulcerative colitis and concurrent primary sclerosing cholangitis. Intest Res. 2012; 10:392–396.

13. Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997; 41:522–525. PMID: 9391253.

14. Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002; 56:48–54. PMID: 12085034.

15. Broomé U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. 2006; 26:31–41. PMID: 16496231.

16. Delaunoit T, Limburg PJ, Goldberg RM, Lymp JF, Loftus EV Jr. Colorectal cancer prognosis among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006; 4:335–342. PMID: 16527697.

17. Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008; 14:378–389. PMID: 18200660.

18. Peyrin-Biroulet L, Lepage C, Jooste V, Guéant JL, Faivre J, Bouvier AM. Colorectal cancer in inflammatory bowel diseases: a population-based study (1976-2008). Inflamm Bowel Dis. 2012; 18:2247–2251. PMID: 22467511.

19. Jensen AB, Larsen M, Gislum M, et al. Survival after colorectal cancer in patients with ulcerative colitis: a nationwide population-based Danish study. Am J Gastroenterol. 2006; 101:1283–1287. PMID: 16771950.

20. Larsen M, Mose H, Gislum M, et al. Survival after colorectal cancer in patients with Crohn's disease: a nationwide population-based Danish follow-up study. Am J Gastroenterol. 2007; 102:163–167. PMID: 17037994.

21. Shu X, Ji J, Sundquist J, Sundquist K, Hemminki K. Survival in cancer patients hospitalized for inflammatory bowel disease in Sweden. Inflamm Bowel Dis. 2011; 17:816–822. PMID: 20645319.

22. Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, Velayos F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012; 143:382–389. PMID: 22609382.

23. Collins PD, Mpofu C, Watson AJ, Rhodes JM. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2006; (2):CD000279. PMID: 16625534.

24. Lutgens MW, Oldenburg B, Siersema PD, et al. Colonoscopic surveillance improves survival after colorectal cancer diagnosis in inflammatory bowel disease. Br J Cancer. 2009; 101:1671–1675. PMID: 19826420.
25. Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010; 138:738–745. PMID: 20141808.

26. Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 1997; 92:204–211. PMID: 9040192.

27. Leighton JA, Shen B, Baron TH, et al. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006; 63:558–565. PMID: 16564852.

28. Eaden JA, Mayberry JF. British Society for Gastroenterology. Association of Coloproctology for Great Britain and Ireland. Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut. 2002; 51(Suppl 5):V10–V12. PMID: 12221032.

29. Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010; 59:666–689. PMID: 20427401.

30. Itzkowitz SH, Present DH. Crohn's and Colitis Foundation of America Colon Cancer in IBD Study Group. Consensus conference: colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005; 11:314–321. PMID: 15735438.

31. Kornbluth A, Sachar DB. Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004; 99:1371–1385. PMID: 15233681.

32. Biancone L, Michetti P, Travis S, et al. European evidence-based consensus on the management of ulcerative colitis: special situations. J Crohns Colitis. 2008; 2:63–92. PMID: 21172196.

33. Ye BD, Yang SK, Boo SJ, et al. Clinical characteristics of ulcerative colitis associated with primary sclerosing cholangitis in Korea. Inflamm Bowel Dis. 2011; 17:1901–1906. PMID: 21830268.

34. Aarnio M, Mustonen H, Mecklin JP, Järvinen HJ. Prognosis of colorectal cancer varies in different high-risk conditions. Ann Med. 1998; 30:75–80. PMID: 9556092.

35. Sugita A, Greenstein AJ, Ribeiro MB, et al. Survival with colorectal cancer in ulcerative colitis. A study of 102 cases. Ann Surg. 1993; 218:189–195. PMID: 8342999.

36. Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004; 126:1634–1648. PMID: 15168373.

37. Hurlstone DP, Sanders DS, Lobo AJ, McAlindon ME, Cross SS. Indigo carmine-assisted high-magnification chromoscopic colonoscopy for the detection and characterisation of intraepithelial neoplasia in ulcerative colitis: a prospective evaluation. Endoscopy. 2005; 37:1186–1192. PMID: 16329015.

38. Kiesslich R, Fritsch J, Holtmann M, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003; 124:880–888. PMID: 12671882.

39. Kiesslich R, Goetz M, Lammersdorf K, et al. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007; 132:874–882. PMID: 17383417.

40. Marion JF, Waye JD, Present DH, et al. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008; 103:2342–2349. PMID: 18844620.

41. Rutter MD, Saunders BP, Schofield G, Forbes A, Price AB, Talbot IC. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004; 53:256–260. PMID: 14724160.

42. Hurlstone DP, Kiesslich R, Thomson M, Atkinson R, Cross SS. Confocal chromoscopic endomicroscopy is superior to chromoscopy alone for the detection and characterisation of intraepithelial neoplasia in chronic ulcerative colitis. Gut. 2008; 57:196–204. PMID: 18192453.

44. Blackstone MO, Riddell RH, Rogers BH, Levin B. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology. 1981; 80:366–374. PMID: 7450425.

45. Ullman T, Croog V, Harpaz N, Sachar D, Itzkowitz S. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003; 125:1311–1319. PMID: 14598247.

46. Befrits R, Ljung T, Jaramillo E, Rubio C. Low-grade dysplasia in extensive, long-standing inflammatory bowel disease: a follow-up study. Dis Colon Rectum. 2002; 45:615–620. PMID: 12004210.
47. Odze RD, Farraye FA, Hecht JL, Hornick JL. Long-term follow-up after polypectomy treatment for adenoma-like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol. 2004; 2:534–541. PMID: 15224277.

48. Riddell RH, Goldman H, Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983; 14:931–968. PMID: 6629368.

49. Torres C, Antonioli D, Odze RD. Polypoid dysplasia and adenomas in inflammatory bowel disease: a clinical, pathologic, and follow-up study of 89 polyps from 59 patients. Am J Surg Pathol. 1998; 22:275–284. PMID: 9500769.
50. Farraye FA, Waye JD, Moscandrew M, Heeren TC, Odze RD. Variability in the diagnosis and management of adenoma-like and non-adenoma-like dysplasia-associated lesions or masses in inflammatory bowel disease: an Internet-based study. Gastrointest Endosc. 2007; 66:519–529. PMID: 17640638.

51. Rutter MD, Saunders BP, Wilkinson KH, Kamm MA, Williams CB, Forbes A. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc. 2004; 60:334–339. PMID: 15332019.

52. Stolfi C, Pellegrini R, Franze E, Pallone F, Monteleone G. Molecular basis of the potential of mesalazine to prevent colorectal cancer. World J Gastroenterol. 2008; 14:4434–4439. PMID: 18680220.

53. Allgayer H. Review article: mechanisms of action of mesalazine in preventing colorectal carcinoma in inflammatory bowel disease. Aliment Pharmacol Ther. 2003; 18(Suppl 2):10–14. PMID: 12950415.

54. Lyakhovich A, Gasche C. Systematic review: molecular chemoprevention of colorectal malignancy by mesalazine. Aliment Pharmacol Ther. 2010; 31:202–209. PMID: 19891667.

55. Moody GA, Jayanthi V, Probert CS, Mac Kay H, Mayberry JF. Long-term therapy with sulphasalazine protects against colorectal cancer in ulcerative colitis: a retrospective study of colorectal cancer risk and compliance with treatment in Leicestershire. Eur J Gastroenterol Hepatol. 1996; 8:1179–1183. PMID: 8980937.

56. Pinczowski D, Ekbom A, Baron J, Yuen J, Adami HO. Risk factors for colorectal cancer in patients with ulcerative colitis: a case-control study. Gastroenterology. 1994; 107:117–120. PMID: 7912678.

57. Nguyen GC, Gulamhusein A, Bernstein CN. 5-aminosalicylic acid is not protective against colorectal cancer in inflammatory bowel disease: a meta-analysis of non-referral populations. Am J Gastroenterol. 2012; 107:1298–1304. PMID: 22751467.

58. van Staa TP, Card T, Logan RF, Leufkens HG. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005; 54:1573–1578. PMID: 15994215.

59. Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000; 14:145–153. PMID: 10651654.

60. Ullman T, Croog V, Harpaz N, et al. Progression to colorectal neoplasia in ulcerative colitis: effect of mesalamine. Clin Gastroenterol Hepatol. 2008; 6:1225–1230. PMID: 18848502.

61. Terdiman JP, Steinbuch M, Blumentals WA, Ullman TA, Rubin DT. 5-Aminosalicylic acid therapy and the risk of colorectal cancer among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007; 13:367–371. PMID: 17206695.

62. Bernstein CN, Blanchard JF, Metge C, Yogendran M. Does the use of 5-aminosalicylates in inflammatory bowel disease prevent the development of colorectal cancer? Am J Gastroenterol. 2003; 98:2784–2788. PMID: 14687833.

63. Choi CH, Kim YH, Kim YS, et al. Guidelines for the management of ulcerative colitis. Intest Res. 2012; 10:1–25.

64. Sebastian S, Hernández V, Myrelid P, et al. Colorectal cancer in inflammatory bowel disease: results of the 3rd ECCO pathogenesis scientific workshop (I). J Crohns Colitis. 2014; 8:5–18. PMID: 23664897.

65. Shetty K, Rybicki L, Brzezinski A, Carey WD, Lashner BA. The risk for cancer or dysplasia in ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol. 1999; 94:1643–1649. PMID: 10364038.

66. Hansen JD, Kumar S, Lo WK, Poulsen DM, Halai UA, Tater KC. Ursodiol and colorectal cancer or dysplasia risk in primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis. Dig Dis Sci. 2013; 58:3079–3087. PMID: 23896754.

67. Matula S, Croog V, Itzkowitz S, et al. Chemoprevention of colorectal neoplasia in ulcerative colitis: the effect of 6-mercaptopurine. Clin Gastroenterol Hepatol. 2005; 3:1015–1021. PMID: 16234048.

68. Lashner BA, Heidenreich PA, Su GL, Kane SV, Hanauer SB. Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis. A case-control study. Gastroenterology. 1989; 97:255–259. PMID: 2568304.
69. Actis GC, Fadda M, Pellicano R, David E, Rizzetto M, Sapino A. The 17-year single-center experience with the use of azathioprine to maintain remission in ulcerative colitis. Biomed Pharmacother. 2009; 63:362–365. PMID: 18657949.

70. Actis GC, Pellicano R, David E, Sapino A. Azathioprine, mucosal healing in ulcerative colitis, and the chemoprevention of colitic cancer: a clinical-practice-based forecast. Inflamm Allergy Drug Targets. 2010; 9:6–9. PMID: 19906011.

71. Baars JE, Looman CW, Steyerberg EW, et al. The risk of inflammatory bowel disease-related colorectal carcinoma is limited: results from a nationwide nested case-control study. Am J Gastroenterol. 2011; 106:319–328. PMID: 21045815.

72. Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005; 54:1121–1125. PMID: 16009685.

73. Kim YJ, Hong KS, Chung JW, Kim JH, Hahm KB. Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev Res (Phila). 2010; 3:1314–1333. PMID: 20736334.

74. Lashner BA, Provencher KS, Seidner DL, Knesebeck A, Brzezinski A. The effect of folic acid supplementation on the risk for cancer or dysplasia in ulcerative colitis. Gastroenterology. 1997; 112:29–32. PMID: 8978339.

75. Biasco G, Zannoni U, Paganelli GM, et al. Folic acid supplementation and cell kinetics of rectal mucosa in patients with ulcerative colitis. Cancer Epidemiol Biomarkers Prev. 1997; 6:469–471. PMID: 9184782.
76. Poynter JN, Gruber SB, Higgins PD, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005; 352:2184–2192. PMID: 15917383.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download