1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon: International Agency for Research on Cancer;2013. cited 2014 Apr 25. Available from:
http://globocan.iarc.fr.
2. Carneiro F. Stomach cancer. In : Steward BW, Wild CP, editors. World Cancer Report 2014. Lyon: International Agency for Research on Cancer;2014. p. 383–391.
3. Ferlay J, Bray F, Steliarova-Foucher E, Forman D. Cancer incidence in five continents, CI5plus: IARC CancerBase No. 9 [Internet]. Lyon: International Agency for Research on Cancer;2014. cited 2014 Apr 25. Available from:
http://ci5.iarc.fr.
4. Forman D, Sierra MS. Introduction: the current and projected global burden of gastric cancer. IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer [Internet]. Lyon: International Agency for Research on Cancer (IARC Working Group Reports, No. 8);2014. cited 2014 Apr 25. p. 5-15. Available from:
http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php.
5. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014; 46:109–123. PMID:
24851102.

6. Choi IJ, Park JY, Herrero R. Effect of Helicobacter pylori eradication on gastric cancer prevention in the Republic of Korea: a randomized controlled clinical trial. IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer [Internet]. Lyon: International Agency for Research on Cancer (IARC Working Group Reports, No. 8);2014. cited 2014 Apr 25. p. 154-160. Available from:
http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php.
7. National Cancer Center. Cancer Facts & Figures 2013 in the Republic of Korea. Goyang: President of National Cancer Center, Minister for Health and Welfare;2013. p. 8.
8. Lauwers GY, Carneiro F, Graham DY, et al. Gastric carcinoma. In : Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. Lyon: International Agency for Research on Cancer;2010. p. 48–58.
9. Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988; 48:3554–3560. PMID:
3288329.
10. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965; 64:31–49. PMID:
14320675.
11. Lauwers GY, Shimizu M, Correa P, et al. Evaluation of gastric biopsies for neoplasia: differences between Japanese and Western pathologists. Am J Surg Pathol. 1999; 23:511–518. PMID:
10328081.
12. Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002; 51:130–131. PMID:
12077106.

13. Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012; 44:74–94. PMID:
22198778.
14. Yada T, Yokoi C, Uemura N. The current state of diagnosis and treatment for early gastric cancer. Diagn Ther Endosc. 2013; 2013:241320. PMID:
23533320.

15. Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000; 47:251–255. PMID:
10896917.

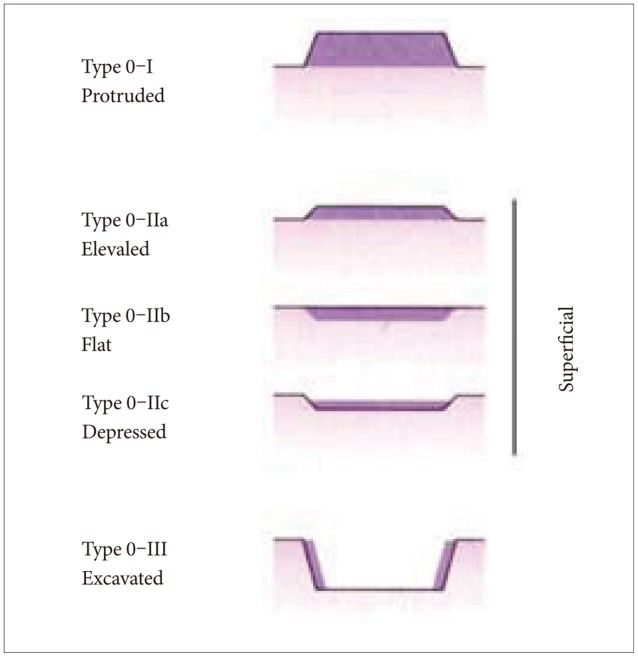
16. Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003; 58(6 Suppl):S3–S43. PMID:
14652541.
17. Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012; 3:251–261. PMID:
22943016.
18. Yoshikawa K, Maruyama K. Characteristics of gastric cancer invading to the proper muscle layer: with special reference to mortality and cause of death. Jpn J Clin Oncol. 1985; 15:499–503. PMID:
2997509.
19. Everett SM, Axon AT. Early gastric cancer in Europe. Gut. 1997; 41:142–150. PMID:
9301490.

20. Yokota T, Kunii Y, Teshima S, et al. Significant prognostic factors in patients with early gastric cancer. Int Surg. 2000; 85:286–290. PMID:
11589593.
21. Kim DY, Joo JK, Ryu SY, Park YK, Kim YJ, Kim SK. Clinicopathologic characteristics of gastric carcinoma in elderly patients: a comparison with young patients. World J Gastroenterol. 2005; 11:22–26. PMID:
15609390.

22. Strong VE, Song KY, Park CH, et al. Comparison of disease-specific survival in the United States and Korea after resection for early-stage node-negative gastric carcinoma. J Surg Oncol. 2013; 107:634–640. PMID:
23192297.

23. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol. 1996; 20:1161–1181. PMID:
8827022.
24. Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007; 56:631–636. PMID:
17142647.

25. Rugge M, Correa P, Di Mario F, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008; 40:650–658. PMID:
18424244.

26. Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010; 71:1150–1158. PMID:
20381801.

27. Carneiro F, Huntsman DG, Smyrk TC, et al. Model of the early development of diffuse gastric cancer in E-cadherin mutation carriers and its implications for patient screening. J Pathol. 2004; 203:681–687. PMID:
15141383.

28. Carneiro F, Oliveira C, Suriano G, Seruca R. Molecular pathology of familial gastric cancer, with an emphasis on hereditary diffuse gastric cancer. J Clin Pathol. 2008; 61:25–30. PMID:
17513507.

29. Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014; 23:700–713. PMID:
24618998.

30. Capelle LG, Van Grieken NC, Lingsma HF, et al. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology. 2010; 138:487–492. PMID:
19900449.

31. Masciari S, Dewanwala A, Stoffel EM, et al. Gastric cancer in individuals with Li-Fraumeni syndrome. Genet Med. 2011; 13:651–657. PMID:
21552135.

32. Song SH, Kim KW, Kim WH, et al. Gastrointestinal cancers in a peutz-jeghers syndrome family: a case report. Clin Endosc. 2013; 46:572–575. PMID:
24143323.

33. Takahashi M, Sakayori M, Takahashi S, et al. A novel germline mutation of the LKB1 gene in a patient with Peutz-Jeghers syndrome with early-onset gastric cancer. J Gastroenterol. 2004; 39:1210–1214. PMID:
15622488.

34. El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000; 404:398–402. PMID:
10746728.

35. Kamangar F, Cheng C, Abnet CC, Rabkin CS. Interleukin-1B polymorphisms and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006; 15:1920–1928. PMID:
17035400.
36. González CA, Megraud F, Buissonniere A, et al. Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project. Ann Oncol. 2012; 23:1320–1324. PMID:
21917738.

37. Lochhead P, El-Omar EM. Helicobacter pylori infection and gastric cancer. Best Pract Res Clin Gastroenterol. 2007; 21:281–297. PMID:
17382277.

38. IARC Working Group. Helicobacter pylori. IARC Working Group. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 100B. Biological Agents. Lyon: International Agency for Research on Cancer;2012. p. 385–436.
39. Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to pylori. Int J Cancer. 2015; 136:487–490. PMID:
24889903.
40. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002; 347:1175–1186. PMID:
12374879.
41. Porras C, Nodora J, Sexton R, et al. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer Causes Control. 2013; 24:209–215. PMID:
23263777.

42. Oona M, Utt M, Nilsson I, Uibo O, Vorobjova T, Maaroos HI. Helicobacter pylori infection in children in Estonia: decreasing seroprevalence during the 11-year period of profound socioeconomic changes. Helicobacter. 2004; 9:233–241. PMID:
15165259.

43. Yim JY, Kim N, Choi SH, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007; 12:333–340. PMID:
17669107.

44. Leja M, Cine E, Rudzite D, et al. Prevalence of Helicobacter pylori infection and atrophic gastritis in Latvia. Eur J Gastroenterol Hepatol. 2012; 24:1410–1417. PMID:
23114744.

45. Peek RM Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006; 208:233–248. PMID:
16362989.

46. Peek RM Jr, Vaezi MF, Falk GW, et al. Role of Helicobacter pylori cagA(+) strains and specific host immune responses on the development of premalignant and malignant lesions in the gastric cardia. Int J Cancer. 1999; 82:520–524. PMID:
10404065.
47. Buti L, Spooner E, Van der Veen AG, Rappuoli R, Covacci A, Ploegh HL. Helicobacter pylori cytotoxin-associated gene A (CagA) subverts the apoptosis-stimulating protein of p53 (ASPP2) tumor suppressor pathway of the host. Proc Natl Acad Sci U S A. 2011; 108:9238–9243. PMID:
21562218.

48. Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003; 301:1099–1102. PMID:
12934009.
49. IARC Working Group. Epstein-Barr virus. IARC Working Group. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 70. Epstein-Barr Virus and Kaposi's Sarcoma Herpesvirus/Human Herpesvirus 8. Lyon: International Agency for Research on Cancer;1997. p. 47–373.
50. Camargo MC, Murphy G, Koriyama C, et al. Determinants of Epstein-Barr virus-positive gastric cancer: an international pooled analysis. Br J Cancer. 2011; 105:38–43. PMID:
21654677.

51. Camargo MC, Kim WH, Chiaravalli AM, et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014; 63:236–243. PMID:
23580779.

52. World Cancer Research Fund. American Institute for Cancer Research. Stomach. World Cancer Research Fund; American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: WCRF/AICR;2007. p. 265–270.
53. Cohen AJ, Roe FJ. Evaluation of the aetiological role of dietary salt exposure in gastric and other cancers in humans. Food Chem Toxicol. 1997; 35:271–293. PMID:
9146740.

54. Furihata C, Ohta H, Katsuyama T. Cause and effect between concentration-dependent tissue damage and temporary cell proliferation in rat stomach mucosa by NaCl, a stomach tumor promoter. Carcinogenesis. 1996; 17:401–406. PMID:
8631123.

55. Tsugane S, Sasazuki S, Kobayashi M, Sasaki S. Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. Br J Cancer. 2004; 90:128–134. PMID:
14710219.

56. Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999; 59:4823–4828. PMID:
10519391.
57. Gaddy JA, Radin JN, Loh JT, et al. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect Immun. 2013; 81:2258–2267. PMID:
23569116.

58. Kato S, Tsukamoto T, Mizoshita T, et al. High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer. 2006; 119:1558–1566. PMID:
16646055.
59. Toyoda T, Tsukamoto T, Hirano N, et al. Synergistic upregulation of inducible nitric oxide synthase and cyclooxygenase-2 in gastric mucosa of Mongolian gerbils by a high-salt diet and Helicobacter pylori infection. Histol Histopathol. 2008; 23:593–599. PMID:
18283644.
60. Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007; 67:4709–4715. PMID:
17510398.

61. Loh YH, Jakszyn P, Luben RN, Mulligan AA, Mitrou PN, Khaw KT. N-Nitroso compounds and cancer incidence: the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Study. Am J Clin Nutr. 2011; 93:1053–1061. PMID:
21430112.

62. Jakszyn P, Bingham S, Pera G, et al. Endogenous versus exogenous exposure to N-nitroso compounds and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study. Carcinogenesis. 2006; 27:1497–1501. PMID:
16571648.
63. Rokkas T, Liatsos C, Petridou E, et al. Relationship of Helicobacter pylori CagA(+) status to gastric juice vitamin C levels. Eur J Clin Invest. 1999; 29:56–62. PMID:
10092990.

64. Zhang ZW, Patchett SE, Perrett D, Katelaris PH, Domizio P, Farthing MJ. The relation between gastric vitamin C concentrations, mucosal histology, and CagA seropositivity in the human stomach. Gut. 1998; 43:322–326. PMID:
9863475.

65. Graham DY, Shiotani A. The time to eradicate gastric cancer is now. Gut. 2005; 54:735–738. PMID:
15888771.

66. Park JY, Forman D, Greenberg ER, Herrero R. Helicobacter pylori eradication in the prevention of gastric cancer: are more trials needed? Curr Oncol Rep. 2013; 15:517–525. PMID:
24101366.

67. Greenberg ER, Park JY. Effectiveness of Helicobacter pylori eradication. IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer [Internet]. Lyon: International Agency for Research on Cancer (IARC Working Group Reports, No. 8);2014. cited 2014 Apr 25. p. 64-71. Available from:
http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php.
68. Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014; 348:g3174. PMID:
24846275.

69. Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012; 104:488–492. PMID:
22271764.

70. Choi J, Kim SG, Yoon H, et al. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol. 2014; 12:793–800. PMID:
24100112.
71. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008; 372:392–397. PMID:
18675689.

72. Herrero R, Parsonnet J, Greenberg ER. Prevention of gastric cancer. JAMA. 2014; 312:1197–1198. PMID:
25247512.

73. IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer [Internet]. Lyon: International Agency for Research on Cancer (IARC Working Group Reports, No. 8);2014. cited 2014 Apr 25. Available from:
http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php.
74. Park JY, Greenberg ER, Parsonnet J, Wild CP, Forman D, Herrero R. Summary of IARC Working Group Meeting on Helicobacter pylori eradication as a strategy for preventing gastric cance. IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer [Internet]. Lyon: International Agency for Research on Cancer (IARC Working Group Reports, No. 8);2014. cited 2014 Apr 25. p. 1-4. Available from:
http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php.
75. Leung WK, Wu MS, Kakugawa Y, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008; 9:279–287. PMID:
18308253.

76. Tsubono Y, Hisamichi S. Screening for gastric cancer in Japan. Gastric Cancer. 2000; 3:9–18. PMID:
11984703.

77. Choi KS, Jun JK, Lee HY, et al. Performance of gastric cancer screening by endoscopy testing through the National Cancer Screening Program of Korea. Cancer Sci. 2011; 102:1559–1564. PMID:
21564421.

78. Hamashima C, Shibuya D, Yamazaki H, et al. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008; 38:259–267. PMID:
18344316.

79. Areia M, Carvalho R, Cadime AT, Rocha Goncalves F, Dinis-Ribeiro M. Screening for gastric cancer and surveillance of premalignant lesions: a systematic review of cost-effectiveness studies. Helicobacter. 2013; 18:325–337. PMID:
23566268.

80. Choi KS, Jun JK, Park EC, et al. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS One. 2012; 7:e50041. PMID:
23209638.

81. Matsumoto S, Ishikawa S, Yoshida Y. Reduction of gastric cancer mortality by endoscopic and radiographic screening in an isolated island: A retrospective cohort study. Aust J Rural Health. 2013; 21:319–324. PMID:
24299436.

82. Riecken B, Pfeiffer R, Ma JL, et al. No impact of repeated endoscopic screens on gastric cancer mortality in a prospectively followed Chinese population at high risk. Prev Med. 2002; 34:22–28. PMID:
11749093.

83. Hamashima C, Ogoshi K, Okamoto M, Shabana M, Kishimoto T, Fukao A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One. 2013; 8:e79088. PMID:
24236091.

84. Lee HY, Park EC, Jun JK, Choi KS, Hahm MI. Comparing upper gastrointestinal X-ray and endoscopy for gastric cancer diagnosis in Korea. World J Gastroenterol. 2010; 16:245–250. PMID:
20066745.

85. Tashiro A, Sano M, Kinameri K, Fujita K, Takeuchi Y. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol. 2006; 12:4873–4874. PMID:
16937471.
86. Nam SY, Choi IJ, Park KW, et al. Effect of repeated endoscopic screening on the incidence and treatment of gastric cancer in health screenees. Eur J Gastroenterol Hepatol. 2009; 21:855–860. PMID:
19369882.

87. Agreus L, Kuipers EJ, Kupcinskas L, et al. Rationale in diagnosis and screening of atrophic gastritis with stomach-specific plasma biomarkers. Scand J Gastroenterol. 2012; 47:136–147. PMID:
22242613.
88. Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006; 9:245–253. PMID:
17235625.

89. Mizuno S, Miki I, Ishida T, et al. Prescreening of a high-risk group for gastric cancer by serologically determined Helicobacter pylori infection and atrophic gastritis. Dig Dis Sci. 2010; 55:3132–3137. PMID:
20204698.

90. Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004; 109:138–143. PMID:
14735480.
91. Zhang X, Xue L, Xing L, et al. Low serum pepsinogen I and pepsinogen I/II ratio and Helicobacter pylori infection are associated with increased risk of gastric cancer: 14-year follow up result in a rural Chinese community. Int J Cancer. 2012; 130:1614–1619. PMID:
21547904.

92. Torres J. The role of biomarkers of gastric cancer risk to target interventions. IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer [Internet]. Lyon: International Agency for Research on Cancer (IARC Working Group Reports, No. 8);2014. cited 2014 Apr 25. p. 122-135. Available from:
http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php.
93. Sipponen P, Ranta P, Helske T, et al. Serum levels of amidated gastrin-17 and pepsinogen I in atrophic gastritis: an observational case-control study. Scand J Gastroenterol. 2002; 37:785–791. PMID:
12190091.
94. Väänänen H, Vauhkonen M, Helske T, et al. Non-endoscopic diagnosis of atrophic gastritis with a blood test. Correlation between gastric histology and serum levels of gastrin-17 and pepsinogen I: a multicentre study. Eur J Gastroenterol Hepatol. 2003; 15:885–891. PMID:
12867799.
95. Leja M, Kupcinskas L, Funka K, et al. Value of gastrin-17 in detecting antral atrophy. Adv Med Sci. 2011; 56:145–150. PMID:
22037174.

96. Tu H, Sun L, Dong X, et al. Temporal changes in serum biomarkers and risk for progression of gastric precancerous lesions: a longitudinal study. Int J Cancer. 2014; 136:425–434. PMID:
24895149.

97. von Karsa L, Qiao YL, Ramadas K, Keita N, Arrossi S, Dean PB. Screening: implementation. In : Stewart BW, Wild CP, editors. World Cancer Report 2014. Lyon: International Agency for Research on Cancer;2014. p. 330–336.
98. Karsa LV, Lignini TA, Patnick J, Lambert R, Sauvaget C. The dimensions of the CRC problem. Best Pract Res Clin Gastroenterol. 2010; 24:381–396. PMID:
20833343.

99. von Karsa L, Dean PB, Arrossi S, Sankaranarayanan R. Screening: principles. In : Stewart BW, Wild CP, editors. World Cancer Report 2014. Lyon: International Agency for Research on Cancer;2014. p. 322–329.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download