Abstract
Metastatic mucinous adenocarcinoma of appendix origin and mimicking a gastric subepithelial tumor (SET) is very rare. Endoscopic ultrasound (EUS)-guided sampling is a useful diagnostic method for SETs. However, the cytologic findings of metastatic mucinous adenocarcinoma are unfamiliar to many pathologists and gastroenterologists. These findings present a diagnostic challenge because the introduction of gastric epithelium and mucin into the specimen during the procedure can be misleading. This is the first reported experience of an EUS-guided sampling of a gastric SET in a patient with suspected appendiceal tumor, to make the diagnosis of a mucinous adenocarcinoma.
Mucinous adenocarcinoma of the appendix can present either with an episode of acute appendicitis or as a mass in the right iliac fossa. It is very rare that the first symptoms are that of metastasis and complications of pseudomyxoma peritonei.1 To our knowledge, there has been no report about a metastatic mucinous adenocarcinoma of appendix origin that mimics a gastric subepithelial tumor (SET). We experienced a case of gastric SET in a patient with suspected appendiceal tumor. We made a preoperative diagnosis of metastatic mucinous adenocarcinoma, on the basis of the result of endoscopic ultrasound (EUS)-guided sampling, through the close cooperation between an endoscopist and a cytopathologist. Herein is a unique case of a metastatic mucinous adenocarcinoma mimicking a gastric SET for which EUS-guided sampling was helpful in making the preoperative diagnosis.
A 64-year-old male patient was referred for the evaluation of gastric SET found incidentally during an upper endoscopy screening. He had a medical history of well-controlled hypertension and diabetes mellitus. Recently, he underwent colonoscopy and upper endoscopy for a health screen. The colonoscopy revealed a 1.5-cm intraluminal protruding lesion with a normal overlying mucosa and tiny polyp on the cecum. The upper endoscopy revealed a dumbbell-shaped SET with shallow linear ulcer on the surface (Fig. 1A, B). A histologic examination of the specimen obtained by forceps biopsy showed only mucosal tissue with nonspecific inflammation at that time. The general physical examination was unremarkable. He denied a history of weight loss, abdominal pain, or melena. The complete blood count and blood chemistry tests were unremarkable.
We performed an abdomen-pelvis computed tomography (CT) to evaluate the SET on the cecum. The CT film showed a 1.8-cm-diameter fluid-filled dilatation of the appendix with no definite periappendiceal fat infiltration, and an ill-defined low attenuated lesion in S6 of the liver. Abdominal ultrasound-guided biopsy revealed that the lesion included fatty change with ballooning degeneration, perivenular fibrosis, sclerosing hyaline necrosis, and portal fibrosis. To better characterize the gastric SET, the patient also underwent a radial EUS (Pentax EG-3670 URK; Pentax, Tokyo, Japan), which revealed that the hypoechoic mass with irregular margins has invaded into the submucosal layers of the stomach and was accompanied with numerous anechoic portions with variable sizes (Fig. 1C). We performed an EUS-guided sampling (Fig. 1D) by using a 22-gauge Echotip Ultra fine-needle biopsy (FNB) needle with ProCore reverse bevel technology (Cook Endoscopy, Limerick, Ireland).
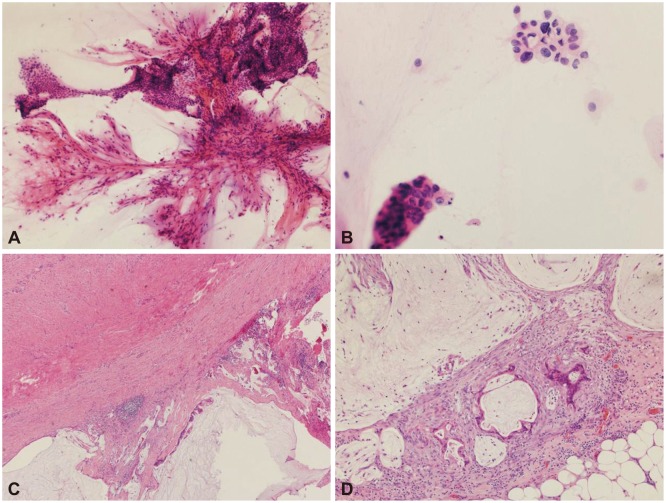
The cytologic results showed abundant mucin with macrophages and a few clusters of mucinous epithelial cells. A few traversing vasculatures were seen within the mucin (Fig. 2A), and although many clusters looked like benign mucinous epithelium, there were a few cells with atypical features such as anisonucleosis, hyperchromasia, and irregular nuclear membrane (Fig. 2B). Metastatic mucinous adenocarcinoma was preoperatively diagnosed on the basis of both imaging studies and cytologic findings. We did not encounter any complications after the EUS-guided sampling. The patient underwent laparotomy (appendectomy, peritoneal biopsy, and omentectomy) to confirm the diagnosis. The appendix measured 5.5 cm in length and 1.6 cm in diameter. On section, the lumen was dilated and filled with thick mucinous material. On microscopic examination, a well-differentiated mucinous adenocarcinoma was seen perforating the visceral peritoneum with high-grade pseudomyxoma peritonei on the serosa (Fig. 2C). The omentum also showed atypical mucinous epithelium with a mucin pool and high-grade pseudomyxoma peritonei (Fig. 2D). Since after the operation, he has been taking a chemotherapy regimen of XELOX (capecitabine and oxaliplatin; Tokyo, Japan) until now.
This case highlights several points. First, primary mucinous adenocarcinoma of the appendix can present with gastric SET. The mechanism of gastrointestinal metastasis includes direct extension from a contiguous or noncontiguous primary malignant tumor, peritoneal carcinomatosis with serosal implants, and embolic metastases with intramural subepithelial masses.2 The presumed mechanism of gastric SET in our patient is that mucinous adenocarcinoma tends to infiltrate through the wall of the appendix and produce multiple tumor deposits on the omentum and peritoneum near the gastric wall, and directly extends into the gastric wall. These are supported by the EUS finding of our patient, which showed a hypoechoic mass with an amorphous shape invading into the gastric wall. Typical sonographic findings of pseudomyxoma peritonei include the anechoic areas in the thickened peritoneum, scalloping of the liver margin, and a "starburst" appearance.3 Anechoic portions with variable sizes were also noted in our patient. It was presumed that the anechoic areas seen in the thickened peritoneum corresponded well to the mucinous nodules produced by the mucinous epithelium.3
Second, EUS-guided sampling can be used as a valuable method in the differential diagnosis of gastric wall metastasis associated with pseudomyxoma peritonei. A few studies have reported that EUS-guided sampling of peritoneal lesions is a safe, minimally invasive, and effective alternative for tissue diagnosis.4,5 The cases diagnosed by using EUS-guided sampling included poorly differentiated adenocarcinoma, pseudomyxoma peritonei, adenocarcinoma, tuberculosis, lymphoma, and breast cancer. The differential diagnosis of peritoneal disease is very broad. Therefore, it is essential to obtain sufficient material suitable for examination with an ancillary method, such as flow cytometry, molecular diagnosis, cytogenetics, or microbiological culture.
Last, the accurate diagnosis of gastric metastasis of pseudomyxoma peritonei can lead to the careful interpretation of EUS-guided sampling under a high index of suspicion. EUS-guided sampling, including EUS-guided fine-needle aspiration or EUS-FNB, is indicated in the following situations:6 SETs with a presumptive diagnosis of unresectable gastrointestinal stromal tumor for which treatment with tyrosine kinase inhibitors is contemplated; a history of malignancy with a SET that may be consistent with a metastasis; and suspected diagnosis of a lymphoma or neuroendocrine tumor on the basis of EUS, or biological or clinical criteria.
Because EUS-guided sampling is done through the stomach or the duodenum, the aspirates usually contain significant amounts of the normal gastric or duodenal epithelium. Although overt malignant cells can be easily differentiated from gastrointestinal contaminants, the neoplastic cells of the low-grade mucinous lesion can be bland or benign-looking, leading to the diagnostic difficulty in differentiating the neoplastic cells from the normal gastrointestinal contaminants. Therefore, the diagnostic challenge in EUS-guided sampling cytology of metastatic low-grade mucinous adenocarcinomas is similar to that of pancreatic cystic lesions such as intraductal papillary mucinous neoplasms and mucinous cystic neoplasms. The difficulty in diagnosing these lesions cytologically is related to both the difficulty in sampling these lesions and the difficulty of properly classifying mucinous cystic lesions, which are commonly composed primarily of cyst fluid with only a small amount of epithelium available for morphologic study.7 An incorrect interpretation of gastrointestinal contamination as a low-grade mucinous neoplasm may result in significant morbidity if the patient undergoes surgical resection.8 On the contrary, delayed diagnosis can be a problem if the low-grade mucinous neoplastic cells are erroneously considered as gastric or duodenal contaminants. Immunohistochemical staining may provide a diagnostic aid in the differential diagnosis because a lack of the cytoplasmic carcinoembryonic antigen expression can be used to identify both gastric and duodenal contaminations. However, the intensity of the immunohistochemical staining can be influenced by several technical factors, and a negative staining of only one antigen can mislead the diagnosis. Only a meticulous examination under a high index of suspicion with the knowledge of the clinical and radiological background of the patient can lead to the correct diagnosis. In our case, features such as traversing vasculature admixed with folded mucinous epithelium and a few mucinous cells with atypical nuclei, as well as the knowledge of the clinical and radiologic setting of the patient, were helpful in reaching the correct diagnosis.
In conclusion, the cytologic findings of the metastatic mucinous adenocarcinoma are unfamiliar to many pathologists and gastroenterologists. However, we believe that an accurate diagnosis of gastric metastasis of mucinous adenocarcinoma from the appendix can be made from EUS-guided sampling cytology, in the appropriate clinicoradiographic context.
References
1. Holland JF, Frei E, Bast RC, Morton DL. Tumours of appendix. In : Holland JF, Frei E, Bast RC, Morton DL, editors. Cancer Medicine. 3rd ed. Philadelphia: Lea & Febiger;1993.
2. Lee NK, Kim S, Kim GH, et al. Hypervascular subepithelial gastrointestinal masses: CT-pathologic correlation. Radiographics. 2010; 30:1915–1934. PMID: 21057127.

3. Que Y, Tao C, Wang X, Zhang Y, Chen B. Pseudomyxoma peritonei: some different sonographic findings. Abdom Imaging. 2012; 37:843–848. PMID: 22234650.

4. Peter S, Eltoum I, Eloubeidi MA. EUS-guided FNA of peritoneal carcinomatosis in patients with unknown primary malignancy. Gastrointest Endosc. 2009; 70:1266–1270. PMID: 19640520.

5. Rana SS, Bhasin DK, Gupta R, Singh K. EUS-guided FNA of peritoneal carcinomatosis. Gastrointest Endosc. 2011; 73:188–189. PMID: 21184887.

6. Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011; 43:897–912. PMID: 21842456.

7. Emerson RE, Randolph ML, Cramer HM. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of intraductal papillary mucinous neoplasm of the pancreas is highly predictive of pancreatic neoplasia. Diagn Cytopathol. 2006; 34:457–462. PMID: 16783773.

8. Toll AD, Bibbo M. Identification of gastrointestinal contamination in endoscopic ultrasound-guided pancreatic fine needle aspiration. Acta Cytol. 2010; 54:245–248. PMID: 20518405.

Fig. 1
Endoscopy, endoscopic ultrasound (EUS), and EUS-guided sampling of the gastric subepithelial tumor (SET). (A) Posterior portion of a dumbbell-shaped SET is noted on the antrum with a normal overlying mucosa. (B) Forceps biopsy is performed in the linear ulcer on the surface of anterior portion of the SET. (C) The hypoechoic mass with an amorphous shape has invaded into the gastric wall layers, and is accompanied with multiple anechoic portions with variable sizes. (D) EUS-guided ProCore needle biopsy is performed on the lesion.
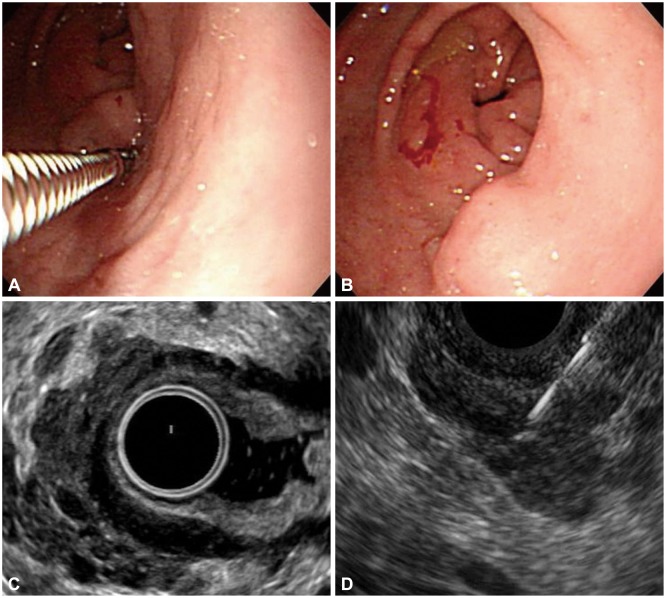
Fig. 2
Pathologic findings of the gastric subepithelial tumor. (A) Lower-power view of the endoscopic ultrasound-guided sampling depicts several branching vasculatures with folded sheets of bland-looking mucinous epithelial cells (H&E stain, ×100). (B) Some of the mucinous cells show nuclear pleomorphism and hyperchromasia, suspicious for malignancy (H&E stain, ×400). (C) The serosa of the resected appendix shows extension of the mucinous adenocarcinoma with several acellular mucin pools (H&E stain, ×40). (D) There are a few glands of metastatic mucinous adenocarcinoma with surrounding inflammation and fibrosis in the omentum. Several acellular mucin polls are also seen (H&E stain, ×100).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download