Abstract
Ectopic pancreas is a congenital anomaly and the most common type of ectopic tissue in the gastrointestinal tract. Most patients with an ectopic pancreas are asymptomatic and rarely have complications. Ectopic pancreatitis after an endoscopic biopsy has not been reported. We report a patient who developed acute ectopic pancreatitis in the stomach after an endoscopic biopsy. A 71-year-old male patient presented with a subepithelial tumor (SET) in the stomach and had no symptoms. Endoscopic ultrasonography demonstrated a 30-mm hypoechoic mural mass, lobulated margins, and anechoic duct-like lesions. To obtain proper tissue specimen, endoscopic biopsy was performed through the opening on the surface of the mass. The pathologic results confirmed an ectopic pancreas. One day after the endoscopic biopsy, he developed persistent epigastric pain. His serum amylase and lipase elevated. Computed tomography of the abdomen showed swelling of the SET and diffuse edema of the gastric wall. His condition was diagnosed as acute ectopic pancreatitis occurring after endoscopic biopsy.
Ectopic pancreas is defined as an abnormally located pancreatic tissue with its own ductal system and vascular supply, and lacking vascular, neural, and anatomical continuity with the normal pancreas.1,2 It is believed to be derived from the displacement of the endoderm that will develop into the pancreas during embryonic development.2 It is incidentally found in about 0.25% of autopsy or surgically resected tissues. Among them, the most common site of discovery is the gastrointestinal tract (90%), including the stomach (24% to 38%), duodenum (9% to 36%), and jejunum (0.5% to 27%).3,4 It is usually located in the submucosal layer (73%); however, it can also be found in the muscular (17%) or subserosal (10%) layers.2,5 Because of its location in the gastric wall, it is difficult to differentiate an ectopic pancreas from other mesenchymal tumors such as gastrointestinal stromal tumors (GISTs).6,7,8 In fact, the pathological diagnosis of an ectopic pancreas is difficult because tissue specimen usually could not be obtained with standard endoscopic biopsy technique.7 Therefore, for an accurate diagnosis, more aggressive endoscopic biopsy techniques such as bite-on-bite biopsy, endoscopic resection, or surgical resection are needed.4,5,6,7,8
Most patients with ectopic pancreas are asymptomatic, and some can have nonspecific symptoms such as abdominal pain or dyspepsia. Complications, including pancreatitis, pseudocysts, gastric outlet obstruction, upper gastrointestinal bleeding, obstructive jaundice, or intussusception, have rarely been reported.9,10 Generally, asymptomatic ectopic pancreas is considered benign, and surgery or endoscopic resection is not necessary.4,5,6,7,8
If a duct opening is present on the surface of the ectopic pancreas, it is possible to obtain adequate tissue specimen by inserting the biopsy forceps through the opening. However, this method can cause mechanical injury to the ectopic pancreatic tissue, and thus there is a possibility that acute pancreatitis will occur. To the best of our knowledge, there has been no report on acute ectopic pancreatitis occurring after an endoscopic biopsy in the gastric ectopic pancreas. Herein, we present a case of acute ectopic pancreatitis occurring after an endoscopic biopsy through the opening of a gastric ectopic pancreas.
A 71-year-old-male patient visited our outpatient clinic because of a subepithelial tumor (SET) in the stomach that was found incidentally during a health check-up endoscopy. He had hypertension and 50-pack-year history of cigarette smoking. On endoscopy, the SET with a nodular shape was found at the lesser curvature in the lower body of the stomach; its surface was covered by normal gastric mucosa, and a small opening was seen at its center (Fig. 1A-C). Endoscopic ultrasonography (EUS) demonstrated an about 30-mm heterogeneously hypoechoic mass in the third, fourth, and fifth layers. Its margins were lobulated and indistinct, and anechoic duct-like lesions were seen inside the mass (Fig. 2). Contrast-enhanced computed tomography (CT) of the abdomen revealed a 30-mm plaque-like mass at the posterior wall of the gastric lower body, and its density was similar to that of the pancreas (Fig. 3A). On the basis of EUS and CT findings, the SET was suspected to be an ectopic pancreas. However, the patient denied the existence of the gastric SET on the previous endoscopy that was performed 1 year before. In addition, he wanted histological confirmation if possible. Therefore, we planned to perform endoscopic biopsy through the opening on the surface of the mass for an accurate diagnosis. When we inserted the biopsy forceps into the opening, the forceps went in deep. Therefore, it was possible to obtain the core tissue inside the tumor; biopsy was performed three times (Fig. 1D).
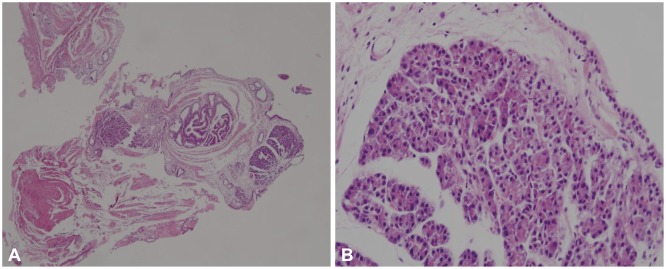
The next day, the patient visited the emergency room because of acute persistent epigastric pain. His vital signs revealed a body temperature of 36.7℃, blood pressure of 130/90 mm Hg, heart rate of 58 beats per minute, and respiratory rate of 20 breaths per minute. Physical examination showed mild epigastric tenderness and mild abdominal distention. Electrocardiography and chest radiography results were unremarkable. Laboratory data included a hemoglobin of 14 g/dL, hematocrit of 37.7%, and white blood cell count of 10,810/µL, aspartate aminotransferase 21 IU/L, alanine aminotransferase 12 IU/L, alkaline phosphatase 215 IU/L, total bilirubin 1.15 mg/dL, calcium 8.6 mg/dL, glucose 115 mg/dL, serum blood urea nitrogen 16 mg/dL, creatinine 0.97 mg/dL, carcinoembryonic antigen 0.92 ng/mL, and carbohydrate antigen 19 to 8.43 U/mL. Elevated levels of serum amylase (241.4 IU/L; reference, 36 to 128 IU/L) and lipase (433.4 IU/L; reference, 22 to 51 IU/L) were observed. On abdominal CT, swelling of the SET and diffuse edema of the gastric wall were observed (Fig. 3B, arrow). The pancreas was normal. When compared with the previous CT image, the tumor became more hypodense than the pancreas. Pathological examination of the endoscopic biopsy specimen revealed that submucosal nodules of normal-appearing pancreatic acinar and ductular structures were separated by bands of fibromuscular tissue (Fig. 4). Therefore, the SET was diagnosed as an ectopic pancreas in the stomach. Finally, it was concluded that acute ectopic pancreatitis occurred after endoscopy biopsy in the gastric ectopic pancreas. The patient was fasted and received intravenous fluids, 40 mg/day pantoprazole, and 600 mg/day gabexate mesilate for 3 days; the abdominal pain improved after 2 days. He was discharged on the sixth day. After discharge, he was followed for 2 years, and symptoms or signs of pancreatitis have not been found thus far.
Ectopic pancreas is commonly encountered in the stomach during endoscopy, and its prevalence is slightly lower than that of GIST, leiomyoma, and schwannoma.11 Malignant transformation from ectopic pancreatic tissue is rare, and it has been reported in about 30 cases of adenocarcinomas.12 Although controversial, invasive treatments such as surgical or endoscopic resection are not recommended for asymptomatic ectopic pancreas with a benign nature.4,5,6,7,8
Ectopic pancreas frequently occurs in the gastric antrum, compared with mesenchymal tumors.12 In the gastric body, GIST or schwannoma are more common.8,13 Therefore, a SET occurring in the gastric body may need endoscopic or surgical resection for the purpose of a differential diagnosis.
Ectopic pancreas located in the gastric body usually appears as a heterogeneously hypoechoic mass in the third, fourth, and fifth layers on EUS. For this reason, pathological diagnosis with standard endoscopic biopsy forceps is difficult.7 Characteristic EUS findings are helpful in diagnosing ectopic pancreas. It may reduce unnecessary surgical or endoscopic resection. It is reported that the characteristic EUS features are indistinct borders, heterogeneous echogenicity, the presence of an anechoic area, involvement of two or more layers, larger longest/shortest ratio, location on the antrum, and mural growth pattern.7,8
The present case showed the above-mentioned characteristic EUS features. Therefore, it could be diagnosed as a gastric ectopic pancreas. However, the patient denied the previous existence of the SET. Although there was a possibility that the lesion was missed during the previous endoscopy, we planned to acquire histological confirmation. In fact, it was possible to obtain proper tissue specimens simply by inserting the biopsy forceps through the opening of its surface, and as a result, the diagnosis of ectopic pancreas was confirmed.
However, the biopsy caused damage to the duct and parenchyma of the ectopic pancreas, which resulted in acute ectopic pancreatitis. This situation is similar to the pancreatitis that occurs after EUS-guided fine needle aspiration (EUS-FNA) of pancreatic lesions. The incidence of acute pancreatitis occurring after EUS-FNA is reported to be rare (0.4%).14 Therefore, although there has been no report on acute ectopic pancreatitis occurring after an endoscopic biopsy, the possibility that it will occur in the gastric ectopic pancreas exits. In fact, we experienced this situation.
In conclusion, we experienced a case of acute ectopic pancreatitis occurring after an endoscopic biopsy through the duct opening of a gastric ectopic pancreas. We suspect that the pathogenesis of ectopic pancreatitis is similar to that of pancreatitis occurring after an EUS-FNA for pancreatic lesions. It is usually recommended that asymptomatic patients with an ectopic pancreas should not undergo invasive procedures such as endoscopic resection. Therefore, when EUS findings strongly suggest that a SET in the stomach is an ectopic pancreas, endoscopic biopsy is usually not needed. If an endoscopic biopsy would be needed to confirm the diagnosis, the biopsy technique of obtaining the pancreatic tissue from the submucosa is desirable. However, in this situation, it should be kept in mind that, although rare, acute ectopic pancreatitis could occur after the biopsy.
References
1. Hsu SD, Wu HS, Kuo CL, Lee YT. Robotic-assisted laparoscopic resection of ectopic pancreas in the posterior wall of gastric high body: case report and review of the literature. World J Gastroenterol. 2005; 11:7694–7696. PMID: 16437703.

2. Lai EC, Tompkins RK. Heterotopic pancreas. Review of a 26 year experience. Am J Surg. 1986; 151:697–700. PMID: 3717502.
3. Osanai M, Miyokawa N, Tamaki T, Yonekawa M, Kawamura A, Sawada N. Adenocarcinoma arising in gastric heterotopic pancreas: clinicopathological and immunohistochemical study with genetic analysis of a case. Pathol Int. 2001; 51:549–554. PMID: 11472568.

4. Tanaka K, Tsunoda T, Eto T, et al. Diagnosis and management of heterotopic pancreas. Int Surg. 1993; 78:32–35. PMID: 8473080.
5. Kaneda M, Yano T, Yamamoto T, et al. Ectopic pancreas in the stomach presenting as an inflammatory abdominal mass. Am J Gastroenterol. 1989; 84:663–666. PMID: 2729238.
6. Chen SH, Huang WH, Feng CL, et al. Clinical analysis of ectopic pancreas with endoscopic ultrasonography: an experience in a medical center. J Gastrointest Surg. 2008; 12:877–881. PMID: 18246404.

7. Park SH, Kim GH, Park do Y, et al. Endosonographic findings of gastric ectopic pancreas: a single center experience. J Gastroenterol Hepatol. 2011; 26:1441–1446. PMID: 21557771.

8. Kim JH, Lim JS, Lee YC, et al. Endosonographic features of gastric ectopic pancreases distinguishable from mesenchymal tumors. J Gastroenterol Hepatol. 2008; 23(8 Pt 2):e301–e307. PMID: 18522684.

9. Armstrong CP, King PM, Dixon JM, Macleod IB. The clinical significance of heterotopic pancreas in the gastrointestinal tract. Br J Surg. 1981; 68:384–387. PMID: 7237066.

10. Mulholland KC, Wallace WD, Epanomeritakis E, Hall SR. Pseudocyst formation in gastric ectopic pancreas. JOP. 2004; 5:498–501. PMID: 15536290.
11. Polkowski M, Butruk E. Submucosal lesions. Gastrointest Endosc Clin N Am. 2005; 15:33–54. PMID: 15555950.

12. Emerson L, Layfield LJ, Rohr LR, Dayton MT. Adenocarcinoma arising in association with gastric heterotopic pancreas: a case report and review of the literature. J Surg Oncol. 2004; 87:53–57. PMID: 15221920.

13. Kwon MS, Lee SS, Ahn GH. Schwannomas of the gastrointestinal tract: clinicopathological features of 12 cases including a case of esophageal tumor compared with those of gastrointestinal stromal tumors and leiomyomas of the gastrointestinal tract. Pathol Res Pract. 2002; 198:605–613. PMID: 12440783.

14. Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011; 73:283–290. PMID: 21295642.

Fig. 1
Endoscopic findings. (A) A subepithelial tumor with a nodular shape is found at the lesser curvature in the lower body of the stomach. (B) On close-up view, a small opening (arrow) is seen at the center. (C) On narrow-band imaging, the tumor is covered by normal gastric mucosa. (D) When the biopsy forceps is inserted into the opening, the forceps goes in deep. Therefore, it is possible to obtain the core tissue inside the tumor.
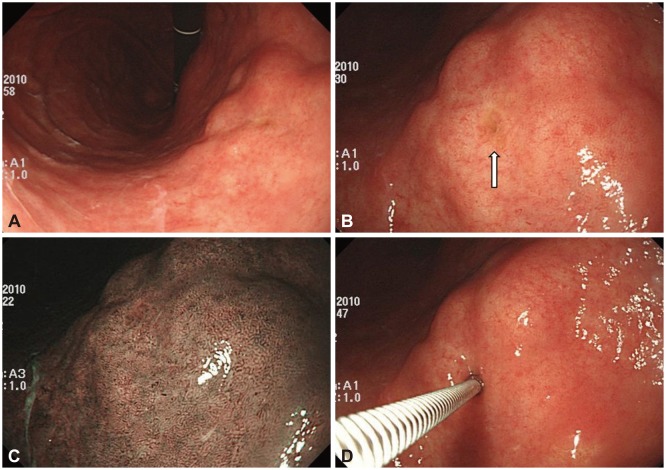
Fig. 2
(A, B) Endoscopic ultrasonography shows an about 30-mm heterogeneously hypoechoic mass in the third, fourth, and fifth layers. Its margins are lobulated and indistinct, and anechoic duct-like structures (arrow) are seen.
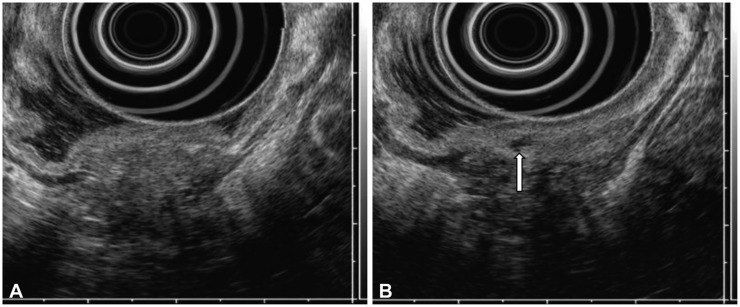
Fig. 3
Abdominal computed tomography (CT) findings. (A) On initial CT, a plaque-like mass (arrow) is seen at the posterior wall of the gastric lower body. (B) On CT during acute pain attack after the endoscopic biopsy, an enlargement of the subepithelial tumor (white arrow) and diffuse gastric wall edema are seen. The pancreas is normal (black arrow). When compared with the previous CT image, its density is more hypodense than that of the pancreas. These findings suggest the occurrence of acute ectopic pancreatitis.
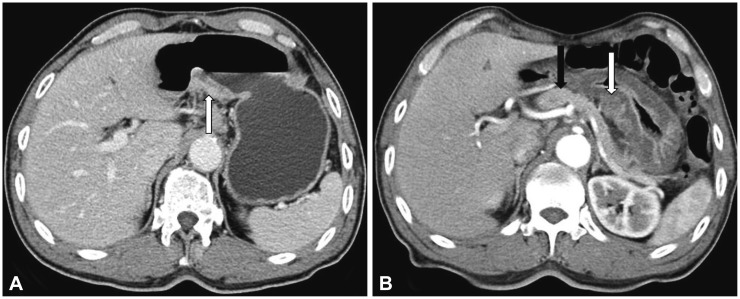
Fig. 4
Pathological findings. (A) Irregularly arranged lobules of pancreatic acinar and dilated ducts are seen in the submucosa (H&E stain, ×40). (B) Acinar cells contain abundant granular eosinophilic cytoplasm in the apical aspect, with basal basophilic cytoplasm. Nuclei are basally located (H&E stain, ×400).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download