Abstract
Endoscopic palliative biliary drainage is considered as a gold standard treatment in advanced or inoperable hilar cholangiocarcinoma. Also, metal stents are preferred over plastic stents in patients with >3 months life expectancy. However, the endoscopic intervention of advanced hilar obstruction is often more challenging and complex than that of distal malignant biliary obstructions. In this literature review, we describe the issues commonly encountered during endoscopic unilateral (single) versus bilateral (multiple) biliary stenting for malignant hilar obstruction. Also, we provide technical guidance to improve the technical success rates and patient outcomes, focusing on bilateral metallic stenting techniques such as stent-in-stent or side-by-side deployment.
Go to : 
Hilar cholangiocarcinoma (HCCA), also known as a Klatskin tumor, is one of the most common types of hepatobiliary cancers, particularly in the Asia-Pacific region, accounting for 46% to 97% of all cholangiocarcinomas.1,2,3,4 However, unfortunately, HCCA has a very poor prognosis, with <10% of patients surviving >5 years after the diagnosis. Typically, only 20% to 30% of HCCA patients are amenable to complete resection at the diagnosis stage.1,5,6,7,8 Regardless, the median survival of patients in whom complete (R0) resection is achieved ranges from only 1 to 4 years, whereas the median survival of patients with unresectable tumors ranges from 5 to 9 months.6,9,10
Multidetector computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP) are the two well-known imaging modalities for the diagnosis and staging of HCCA, as well as for determining its resectability.1 Because most HCCA patients are not good candidates for curative resection, palliative biliary decompression is achieved through endoscopic drainage by using metal or plastic stents, or by means of biliary-enteric bypass surgery and transhepatic percutaneous drainage.11,12,13 When appropriate endoscopic expertise is available, endoscopic palliation may be considered the treatment of choice for nonresectable HCCA. If an endoscopic approach fails to resolve obstructive cholestasis, rendezvous percutaneous and endoscopic procedures or percutaneous drainage alone may be used for the palliation of HCCA.11
Although endoscopic palliation is preferred, there is no definite consensus about the optimal approach. Controversy still exists about the benefits of using one or multiple stents (unilateral vs. bilateral stenting), and whether plastic or metal stents should be preferred. In addition, biliary decompression is often more challenging and complex in HCCA than in distal malignant biliary obstructions, with a lower clinical success rate. Recently, the Asia-Pacific Working Group on hepatobiliary cancers provided several recommendations concerning biliary drainage in the treatment of HCCA.1 First, in Bismuth type II to IV HCCA patients with a predicted survival of >3 months, metal stents are superior to plastic stents with respect to both patient outcomes and cost-effectiveness. Second, for the palliation of advanced HCCA such as Bismuth III or IV tumors, percutaneous stenting or drainage shows superior outcomes to those of endoscopic palliation. Third, the goal of palliative stenting is drainage of an adequate liver volume (>50%), irrespective of using unilateral, bilateral, or multisegmental stenting.
Various endoscopic or other palliative methods such as chemotherapy with/without radiotherapy, or photodynamic therapy, have been developed to provide symptomatic relief and prolong stent patency and survival. Here, we review the literature about the current strategies and technical issues encountered during endoscopic biliary drainage, focusing on bilateral metallic stenting in advanced and inoperable HCCA.
Go to : 
More than 90% of nonresectable HCCAs involve obstructive cholestasis and, therefore, restoration of bile flow is one of the main objectives of palliative treatment. Currently, there is no definitive consensus on the best endoscopic method when performing bilateral vs. unilateral stent placement (or single vs. multiple stenting) in patients with advanced HCCA such as Bismuth type II or higher tumors. The decision on whether to place single or multiple biliary stents depends on the extent of malignant biliary stricture and on the degree of biliary tract contamination.11 A single biliary stent in one dominant functional liver lobe (i.e., unilateral drainage) can provide adequate palliation in most patients with HCCA. The primary factor associated with effective drainage is the liver volume drained. It is well known that biliary drainage of >25% of the liver volume is required for adequate palliation of obstructive cholestasis and to improve biochemical parameters.14 Approximately 55% to 60% of the liver volume is drained through the right hepatic duct, 30% to 35% through the left hepatic duct, and 10% from the caudate lobe. However, there was no significant difference in terms of successful drainage, complications, number of endoprosthesis changes, or survival among patients with right-duct drainage compared with those undergoing left-side drainage.15 In a recent study, Vienne et al.16 analyzed the factors predicting drainage effectiveness during endoscopic stenting for malignant hilar biliary strictures. The main factor associated with effective drainage was the volume of the liver drained (>50%), as assessed by using CT, and this was especially important in Bismuth III strictures. Moreover, >50% drainage was associated with prolonged survival in treated patients (119 days) compared with patients with <50% drainage (59 days, p=0.005). Therefore, to achieve a drainage volume of >50% and its associated benefits, bilateral or multiple drainage may be warranted.
Recently, the Asia-Pacific Working Group for hepatobiliary cancers also stated that palliative stenting in HCCA patients should aim to achieve an adequate drainage of 50% or more of the total liver volume, irrespective of whether unilateral, bilateral, or multisegmental stenting is used. However, for the adequate drainage of >50% of the liver volume, the use of more than one stent, either bilateral or multisegmental, depending on the patient's anatomy, is commonly required.1 In addition, atrophied segments and aberrant ductal anatomy need to be assessed with noninvasive imaging before attempting biliary drainage.17 In Bismuth type II tumors, bilateral endoscopic drainage is preferred if drainage of 50% of the liver volume cannot be achieve with a single stent. However, in Bismuth type III/IV tumors, bilateral or multisegmental drainage through the percutaneous approach is preferred over the endoscopic approach. The advantage of the percutaneous approach is its ability to achieve precise lobar selection for drainage. However, percutaneous drainage is uncomfortable to patients and may need a two-step process. When experienced endoscopic experts are available, the endoscopic approach may also be a first choice even in advanced hilar obstruction.
Technically, single stent insertion to alleviate hilar obstruction is a safe and simple method. De Palma et al.18,19 revealed that unilateral metal stent drainage is safe, easy, and achieves adequate drainage in most patients with nonresectable HCCA. Technical success was achieved in 96.7% (59 of 61), with stent malfunction occurring in only three cases (4.9%). Functionally successful drainage was achieved in 96.7% (59 of 61), and complete resolution of jaundice occurred in 86%. Compared with bilateral stents, unilateral stents showed a significantly higher rate of successful endoscopic insertion (76.9% vs. 88.6%, respectively; p=0.041) and a significantly lower rate of complications (26.9% vs. 18.9%, respectively; p=0.026). Therefore, the authors concluded that the insertion of more than one endoprosthesis would not be justified as a routine procedure in patients with hilar tumors. Unilateral stenting with either metal or plastic stents showed a significantly higher rate of technical success, lower complication rate, and higher rate of successful drainage compared with bilateral stent insertion.15,18,19,20,21 Iwano et al.21 also reported that unilateral drainage is associated with a lower incidence of liver abscess, as well as comparable outcomes with regard to stent patency and complication-free survival compared with bilateral drainage. Recently, Mukai et al.22 reported that there was no significant difference in the stent patency between unilateral and bilateral stenting. However, reintervention for stent dysfunction is more complicated in bilateral stenting. The success rate for endoscopic reintervention was significantly higher in unilateral stenting than in bilateral stenting (100% vs. 68%; p=0.0272). They concluded that unilateral stenting is more beneficial than bilateral stenting.
Nevertheless, despite being a simple and safe method, unilateral drainage may not provide adequate drainage physiologically and does not show superior stent patency.
Although bilateral or multiple biliary drainage is controversial, it may be necessary specifically when both hepatic lobes are diseased or opacified, when a nondominant or atrophic lobe has been inadvertently stented without achieving drainage, or if bilateral brachytherapy is scheduled. Chang et al.23 observed the highest survival among HCCA patients who underwent bilateral drainage (225 days), and markedly lower survival among those who showed cholangiographic filling of both lobes (i.e., opacification) but underwent drainage on only one side (80 days, p<0.01). In addition, the authors suggested that inadvertent contrast medium injection into the intrahepatic ducts without adequate drainage was associated with worsened outcomes. In addition to ensuring adequate drainage, bilateral stenting may improve stent patency. Naitoh et al.24 found no significant difference between unilateral and bilateral endoscopic metal stenting with regard to successful insertion (100% vs. 90%), successful drainage (100% vs. 96%), incidence of early complications (0% vs. 10%), or incidence of late complications (65% vs. 54%). However, the cumulative stent patency was higher in the bilateral stent group (median stent patency, 488 days) than in the unilateral stent group (210 days, p=0.009). Liberato and Canena25 reported that endoscopic reintervention for stent occlusion was required more frequently in patients with unilateral rather than bilateral plastic stents (80.9% vs. 34.2%, respectively; p<0.001) and in those with unilateral rather than bilateral self-expanding metal stent (SEMS) (31.4% vs. 11.9%, respectively; p=0.036). The median stent patency period was 17 weeks for unilateral plastic stents, 18 weeks for bilateral plastic stents, 24 weeks for unilateral SEMS, and 29 weeks for bilateral SEMS. Multivariate analysis revealed that SEMS placement and bilateral deployment were the only independent prognostic factors associated with prolonged stent patency.
Accordingly, bilateral or multiple drainage may be more physiologically sound than unilateral drainage. Also, various bilateral drainage techniques and newly developed metal stents are now available.26,27,28,29,30,31 Nevertheless, the deployment of multiple metal stents for bilateral drainage is generally thought to be technically demanding. One commonly encountered surgical error is the inadvertent injection of contrast medium past the hilar stricture and into atrophied and/or unintended multiple hepatic segments, which may lead to postprocedure cholangitis and lower survival rates.23 In addition, the incidence of cholangitis in patients with hilar obstruction was significantly higher than in those with a distal obstruction.32 To reduce the incidence of postendoscopic retrograde cholangiopancreatography (ERCP) cholangitis, several studies have examined the use of selectively targeted and planned endoscopic drainage guided by MRCP or CT imaging, and found a decreased incidence of postprocedure complications.19,31,32,33,34,35 Thus, when performed correctly, endoscopic bilateral deployment of multiple metal stents has been shown to be a feasible and highly useful procedure, particularly in terms of prolonging patency and preserving functional liver volume.36 A multicenter retrospective study reported that successful biliary drainage was significantly higher in the percutaneous group than in the endoscopic group (93% vs. 77%; p=0.049).37 However, institutional expertise in endoscopy may also be an important factor.
When viewed from the perspective of an experienced endoscopist, bilateral drainage by using metal stents may be a technically feasible and safe method. Numerous recent studies also revealed higher technical and functional success rates in bilateral drainage even with metallic stents.14,26,27,28,29,38,39,40,41,42 Accordingly, if appropriate endoscopic expertise is available, endoscopic palliation may be the first choice even for advanced HCCA, Bismuth type III or IV strictures.
Go to : 
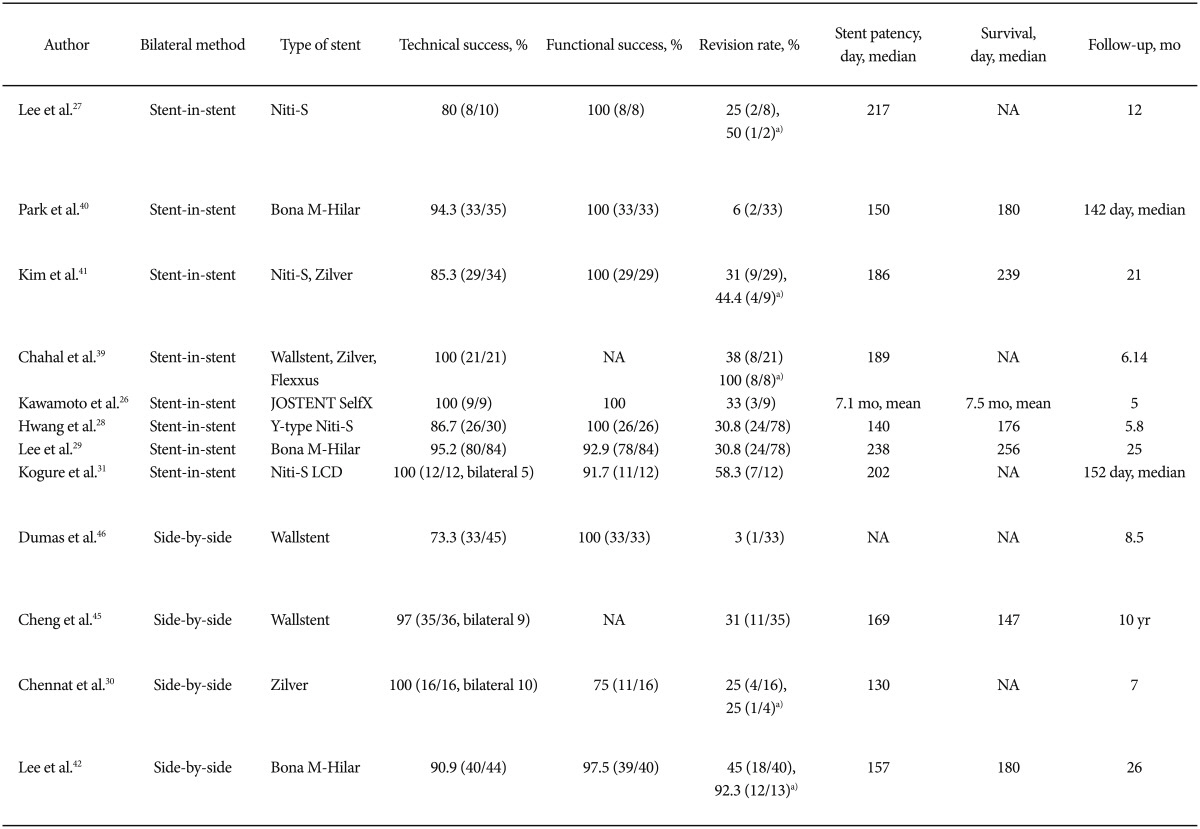
Bilateral biliary drainage with metal stents for HCCA can be performed by using one of two methods. After setting the first stent in the intrahepatic duct in one segment, a second stent is placed through the previously inserted stent by using the "stent-in-stent" method, which involves crossing through the mesh within the initial stent.11,43 Alternatively, the second stent can be placed parallel to the first stent (the "side-by-side" method). The reported technical success rates for experts using the stent-in-stent or side-by-side procedures range from 73.3% to 100% (Table 1). However, bilateral stents are associated with technical difficulty and an increased risk of complications when multiple drainage failed after contrast injection. As a result, some endoscopists may hesitate to insert bilateral stents, particularly metal stents. In the small number of case studies comparing side-by-side and stent-in-stent deployment of a SEMS for malignant hilar obstruction, it was shown that the incidence of complications was higher in side-by-side procedures. However, side-by-side deployment tends to result in higher stent patency rates compared with stent-in-stent deployment.44
Bilateral side-by-side stent placement for HCCA is frequently performed by using an endoscopic approach and a parallel arrangement, as reported by Cheng et al.45 and Dumas et al.46 If both guidewires are inserted into the right and left intrahepatic ducts, sequential stent insertion may be easy. In addition, bilateral revision when stents become occluded may be easier because endoscopic revision can be performed through each stent. The issues surrounding this technique include the potential entanglement of the two guidewires, the difficulty of precisely deploying the stent to ensure adequate drainage, and the need for endoscopic revision should the stents become occluded.11 After the deployment of the first SEMS, insertion of the delivery system of the second stent can be disturbed by the resistance against the first deployed SEMS. Insertion after preloading on the guidewire may speed up the procedure, and small-diameter delivery systems with good pushability are needed. Both SEMS should be placed with their distal ends in the duodenum or at the same level in the common bile duct to facilitate SEMS revision when the stents occlude. However, precise deployment of both stents on the same level may be difficult in cases of different levels of stricture length. To overcome these difficulties, Chennat and Waxman30 introduced a simultaneous side-by-side deployment method by using a thin 6-Fr delivery system. This system showed a high rate of technical success, and it may be feasible and ideal for use in simultaneous side-by-side deployment. However, the handling of a side-by-side simultaneous delivery catheter within the same endoscopic working channel and biliary duct resulted in additional frictional forces, which may increase the tendency of the delivery system to buckle in the distal duct. Also, the resulting median stent patency was relatively short (130 days), which might be due to the short follow-up period and the small diameter of stents. Additional large-scale studies with long-term follow-up are needed.
Technically, the use of a guidewire to cannulate to the desired contralateral bile duct through the previously inserted SEMS may be difficult in bilateral stent-in-stent procedures. In addition, during endoscopic revision for stent occlusion due to tumor ingrowth, the previously inserted wire mesh may preclude the insertion of a revisionary stent. To enhance secondary SEMS insertion, balloon dilation of the contralateral hepatic duct immediately before the first stenting, or dilation of the first deployed SEMS with a balloon before inserting the contralateral second SEMS, can be useful.29,40,42 In cases of severe stricture, following the expansion of the first stent, delayed insertion of the second stent by 2 to 3 days may also be helpful.
Structurally, large open-celled wire mesh stents can improve the technical success rates in cases of severe stricture. Kogure et al.31 (Niti-S large cell D-type; Taewoong Corp., Seoul, Korea) and Lee et al.27 and Hwang et al.28 (Niti-S Biliary Y-stent; Taewoong) showed high technical success rates with large-cell stents in the bilateral stent-in-stent method, although the number of cases in each study was small. However, the expanding radial force may decrease in stricture sites and can create an inherent susceptibility to tumor ingrowth because of their large open-mesh design. In addition, full expansion of the second stent may be hindered owing to the space limitation moving through the large open mesh. Alternatively, closed-cell, cross-wired metal stents, such as the M-hilar Bonastent (Standard Sci Tech Inc., Seoul, Korea) also showed high technical success and revision efficacy for endoscopic bilateral stent-in-stent placement in HCCA patients. The primary technical success rate of endoscopic bilateral stent-in-stent placement was 95.2%, and the median patency was 238 days. The technical and clinical success rates of planned bilateral endoscopic revision for occluded stents were 83.3% (20 of 24) and 79.2% (19 of 24), respectively.29 Endoscopic bilateral revision with plastic or metal stents was not restricted technically.
The reported success rate of endoscopic revisions in bilateral metal stents varied from 44.4% to 100%, as shown in Table 1. However, the reported studies used various combinations of unilateral or bilateral stent insertion by means of side-by-side or stent-in-stent insertion methods. Furthermore, the limited number of patients and conflicting data among these studies prohibit a direct comparison of the results. Nevertheless, newly developed or modified stents and devices recently showed higher technical feasibility without increased complications. These results may encourage ERCP endoscopists to use these techniques and stents as soon as possible to prevent difficulties.
Well-designed, large-scaled comparative studies comparing stent materials and deployment methods in HCCA are now limited. Direct comparison of reported studies is made difficult by the variety of stents and methods used. As long as experienced endoscopists are available, bilateral drainage by using SEMS may be the preferred option, whether placed by means of the stent-in-stent or the side-by-side deployment method. However, it is still controversial which method is better. Also, for optimal drainage, multisectoral drainage rather than unilateral or bilateral drainage should be considered regardless of the use of the endoscopic or percutaneous approach in advanced hilar obstruction. Endoscopists' preferences and institutional capabilities should also be considered before the procedure. Also, besides endoscopic palliation, additional therapies such as chemotherapy, radiotherapy, photodynamic therapy, and the recently reported radiofrequency ablation for the local treatment of tumors should also be considered for improving patency and patient survival. Future prospective multicenter studies are needed to establish the optimal therapeutic protocol.
Go to : 
Endoscopic palliation of advanced HCCA remains challenging for endoscopists. For effective endoscopic biliary drainage, it is important to select the appropriate stent according to the patient's condition and the anatomical position of the lesion. Then, palliative therapeutic strategies should be designed on a patient basis, with consideration given to available endoscopy expertise, patient condition, and existing medical resources. For the optimal physiological drainage, multisectoral drainage may be needed in advanced HCCA. Further clinical and technical refinement is expected through additional randomized, controlled trials evaluating these therapeutic issues and new technologies. Finally, to prolong stent patency and patient survival, additive therapeutic options should be considered according to the patient's condition and availability.
Go to : 
References
1. Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013; 28:593–607. PMID: 23350673.

2. Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011; 8:512–522. PMID: 21808282.

3. Deoliveira ML, Schulick RD, Nimura Y, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011; 53:1363–1371. PMID: 21480336.

4. Yusoff AR, Siti ZM, Muzammil AR, Yoong BK, Vijeyasingam R. Cholangiocarcinoma: a 10-year experience of a single tertiary centre in the multi ethnicity-Malaysia. Med J Malaysia. 2012; 67:45–51. PMID: 22582548.
5. Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009; 208:134–147. PMID: 19228515.

6. Witzigmann H, Berr F, Ringel U, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg. 2006; 244:230–239. PMID: 16858185.
7. Otani K, Chijiiwa K, Kai M, et al. Outcome of surgical treatment of hilar cholangiocarcinoma. J Gastrointest Surg. 2008; 12:1033–1040. PMID: 18085342.

8. Li H, Qin Y, Cui Y, Chen H, Hao X, Li Q. Analysis of the surgical outcome and prognostic factors for hilar cholangiocarcinoma: a Chinese experience. Dig Surg. 2011; 28:226–231. PMID: 21540611.

9. Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005; 241:693–699. PMID: 15849505.

10. Nishio H, Nagino M, Nimura Y. Surgical management of hilar cholangiocarcinoma: the Nagoya experience. HPB (Oxford). 2005; 7:259–262. PMID: 18333203.

11. Kim JH. Endoscopic stent placement in the palliation of malignant biliary obstruction. Clin Endosc. 2011; 44:76–86. PMID: 22741117.

12. Raijman I. Biliary and pancreatic stents. Gastrointest Endosc Clin N Am. 2003; 13:561–592. PMID: 14986787.

13. Wagner HJ, Knyrim K, Vakil N, Klose KJ. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. A prospective and randomized trial. Endoscopy. 1993; 25:213–218. PMID: 7686100.

14. Dowsett JF, Vaira D, Hatfield AR, et al. Endoscopic biliary therapy using the combined percutaneous and endoscopic technique. Gastroenterology. 1989; 96:1180–1186. PMID: 2925062.

15. Polydorou AA, Chisholm EM, Romanos AA, et al. A comparison of right versus left hepatic duct endoprosthesis insertion in malignant hilar biliary obstruction. Endoscopy. 1989; 21:266–271. PMID: 2482169.

16. Vienne A, Hobeika E, Gouya H, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. 2010; 72:728–735. PMID: 20883850.

17. Kwon AH, Uetsuji S, Ogura T, Kamiyama Y. Spiral computed tomography scanning after intravenous infusion cholangiography for biliary duct anomalies. Am J Surg. 1997; 174:396–401. discussion 401-392. PMID: 9337161.

18. De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001; 53:547–553. PMID: 11323577.

19. De Palma GD, Pezzullo A, Rega M, et al. Unilateral placement of metallic stents for malignant hilar obstruction: a prospective study. Gastrointest Endosc. 2003; 58:50–53. PMID: 12838220.

20. Sherman S. Endoscopic drainage of malignant hilar obstruction: is one biliary stent enough or should we work to place two? Gastrointest Endosc. 2001; 53:681–684. PMID: 11323609.

21. Iwano H, Ryozawa S, Ishigaki N, et al. Unilateral versus bilateral drainage using self-expandable metallic stent for unresectable hilar biliary obstruction. Dig Endosc. 2011; 23:43–48.

22. Mukai T, Yasuda I, Nakashima M, et al. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2013; 20:214–222. PMID: 22415652.

23. Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998; 47:354–362. PMID: 9609426.

24. Naitoh I, Ohara H, Nakazawa T, et al. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol. 2009; 24:552–557. PMID: 19220678.

25. Liberato MJ, Canena JM. Endoscopic stenting for hilar cholangiocarcinoma: efficacy of unilateral and bilateral placement of plastic and metal stents in a retrospective review of 480 patients. BMC Gastroenterol. 2012; 12:103. PMID: 22873816.

26. Kawamoto H, Tsutsumi K, Fujii M, et al. Endoscopic 3-branched partial stent-in-stent deployment of metallic stents in high-grade malignant hilar biliary stricture (with videos). Gastrointest Endosc. 2007; 66:1030–1037. PMID: 17963891.
27. Lee JH, Kang DH, Kim JY, et al. Endoscopic bilateral metal stent placement for advanced hilar cholangiocarcinoma: a pilot study of a newly designed Y stent. Gastrointest Endosc. 2007; 66:364–369. PMID: 17643714.
28. Hwang JC, Kim JH, Lim SG, Kim SS, Yoo BM, Cho SW. Y-shaped endoscopic bilateral metal stent placement for malignant hilar biliary obstruction: prospective long-term study. Scand J Gastroenterol. 2011; 46:326–332. PMID: 21082874.

29. Lee TH, Moon JH, Kim JH, et al. Primary and revision efficacy of cross-wired metallic stents for endoscopic bilateral stent-in-stent placement in malignant hilar biliary strictures. Endoscopy. 2013; 45:106–113. PMID: 23212727.

30. Chennat J, Waxman I. Initial performance profile of a new 6F self-expanding metal stent for palliation of malignant hilar biliary obstruction. Gastrointest Endosc. 2010; 72:632–636. PMID: 20579991.

31. Kogure H, Isayama H, Nakai Y, et al. Newly designed large cell Niti-S stent for malignant hilar biliary obstruction: a pilot study. Surg Endosc. 2011; 25:463–467. PMID: 20602139.

32. Nomura T, Shirai Y, Hatakeyama K. Cholangitis after endoscopic biliary drainage for hilar lesions. Hepatogastroenterology. 1997; 44:1267–1270. PMID: 9356838.
33. Khan SA, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002; 51(Suppl 6):VI1–VI9. PMID: 12376491.

34. Freeman ML, Overby C. Selective MRCP and CT-targeted drainage of malignant hilar biliary obstruction with self-expanding metallic stents. Gastrointest Endosc. 2003; 58:41–49. PMID: 12838219.

35. Hintze RE, Abou-Rebyeh H, Adler A, Veltzke-Schlieker W, Felix R, Wiedenmann B. Magnetic resonance cholangiopancreatography-guided unilateral endoscopic stent placement for Klatskin tumors. Gastrointest Endosc. 2001; 53:40–46. PMID: 11154487.

36. Kato H, Tsutsumi K, Harada R, Okada H, Yamamoto K. Endoscopic bilateral deployment of multiple metallic stents for malignant hilar biliary strictures. Dig Endosc. 2013; 25(Suppl 2):75–80. PMID: 23617654.

37. Paik WH, Park YS, Hwang JH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. 2009; 69:55–62. PMID: 18657806.

38. Polydorou AA, Cairns SR, Dowsett JF, et al. Palliation of proximal malignant biliary obstruction by endoscopic endoprosthesis insertion. Gut. 1991; 32:685–689. PMID: 1711994.

39. Chahal P, Baron TH. Expandable metal stents for endoscopic bilateral stent-within-stent placement for malignant hilar biliary obstruction. Gastrointest Endosc. 2010; 71:195–199. PMID: 19945101.

40. Park do H, Lee SS, Moon JH, et al. Newly designed stent for endoscopic bilateral stent-in-stent placement of metallic stents in patients with malignant hilar biliary strictures: multicenter prospective feasibility study (with videos). Gastrointest Endosc. 2009; 69:1357–1360. PMID: 19481654.
41. Kim JY, Kang DH, Kim HW, et al. Usefulness of slimmer and open-cell-design stents for endoscopic bilateral stenting and endoscopic revision in patients with hilar cholangiocarcinoma (with video). Gastrointest Endosc. 2009; 70:1109–1115. PMID: 19647244.

42. Lee TH, Park do H, Lee SS, et al. Technical feasibility and revision efficacy of the sequential deployment of endoscopic bilateral side-by-side metal stents for malignant hilar biliary strictures: a multicenter prospective study. Dig Dis Sci. 2013; 58:547–555. PMID: 22886596.

43. Lee TH. Technical tips and issues of biliary stenting, focusing on malignant hilar obstruction. Clin Endosc. 2013; 46:260–266. PMID: 23767037.

44. Naitoh I, Hayashi K, Nakazawa T, et al. Side-by-side versus stent-in-stent deployment in bilateral endoscopic metal stenting for malignant hilar biliary obstruction. Dig Dis Sci. 2012; 57:3279–3285. PMID: 22732832.

45. Cheng JL, Bruno MJ, Bergman JJ, Rauws EA, Tytgat GN, Huibregtse K. Endoscopic palliation of patients with biliary obstruction caused by nonresectable hilar cholangiocarcinoma: efficacy of self-expandable metallic Wallstents. Gastrointest Endosc. 2002; 56:33–39. PMID: 12085032.

46. Dumas R, Demuth N, Buckley M, et al. Endoscopic bilateral metal stent placement for malignant hilar stenoses: identification of optimal technique. Gastrointest Endosc. 2000; 51:334–338. PMID: 10699784.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download