Abstract
Molecular imaging in gastroenterology has become more feasible with recent advances in imaging technology, molecular genetics, and next-generation biochemistry, in addition to advances in endoscopic imaging techniques including magnified high-resolution endoscopy, narrow band imaging or autofluorescence imaging, flexible spectral imaging color enhancement, and confocal laser endomicroscopy. These developments have the potential to serve as "red flag" techniques enabling the earlier and accurate detection of mucosal abnormalities (such as precancerous lesions) beyond biomarkers, virtual histology of detected lesions, and molecular targeted therapy-the strategy of "one stone to kill two or three birds"; however, more effort should be done to be "blue ocean" benefit. This review deals with the introduction of Raman spectroscopy endoscopy, imaging mass spectroscopy, and nanomolecule development for theranostics. Imaging of molecular pathological changes in cells/tissues/organs might open the "royal road" to either convincing diagnosis of diseases that otherwise would only be detected in the advanced stages or novel therapeutic methods targeted to personalized medicine.
As molecular imaging has the very advantage of being capable of mapping the in situ locations of specific molecules of interest within live tissue, it has shown enormous potential as a powerful way to either diagnose or monitor clinical diseases much earlier before an emergency or overt clinical manifestation.1 Currently, positron emission tomography (PET), PET-computed tomography (PET-CT), single photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI) are already widely used in the clinic, imparting considerable contributions to patient care with valuable pathogenic information that could not be obtained with traditional ways of visualization. In detail, nuclear medicine applications use devices such SPECT and PET for two basic functions: first as a diagnostic imaging tool and second as a monitoring tool during therapy.2,3 The desire to evaluate underlying pathologies beyond better visualizations have prompted the development of newer endoscopy devices equipped with new optical technologies, such as an endoscope with narrow band imaging (NBI), which enhances the accuracy of diagnosis by using narrow-bandwidth filters in a red-green-blue sequential illumination system; autofluorescence imaging (AFI), which is based on the detection of natural tissue fluorescence emitted by endogenous molecules; and the i-Scan technology, which uses a digital filter that modifies normal images through software functions. Flexible spectral imaging color enhancement of detailed mucosal structure and microcirculation condition, and confocal laser endomicroscopy (CLE) images collected with an argon beam are already available.4 However, the avidity for endoscopy that enables imaging biochemical pathways combined with spectroscopy imaging, just like fingerprinting technology to identify a person, is currently an issue of intensive research, offering future perspectives on early detection and accurate diagnosis even before overt clinical manifestations or a visual endoscopic diagnosis. Therefore, molecular imaging is aimed at diagnosing precancerous stages far before a visual diagnosis, monitoring treatment efficiency, and even predicting prognosis with optimized therapy. As parts of these technologies, in this review article, the advancements in Raman spectroscopy (RS), imaging mass spectroscopy (IMS), and theranostic namomedicine will be described with the introduction of some achievements from our group in this field.
RS is an optical technique developed almost 50 years ago by Dr. Raman, a Nobel laureate. It has been used predominantly as an analytic tool for routine chemical composition, on the basis of its unsurpassed sensitivity and multiplexing capabilities, together with its ability to explore biomolecules in a nondestructive and nonlabeling manner. Researchers have been able to harness its unique properties for imaging, and RS has provided accurate spectral information about molecular characteristics as well as more detailed interactions in either the cell level or in preclinical animal models. Furthermore, basic and clinical researchers have translated this optical technique into a novel clinical diagnostic tool, by which various endoscopic strategies were further improved with the use of nanoparticles capable of compensating for weak Raman signals and for the longer time of acquisition. Lastly, recent advances in imaging devices, such as the development of slit-scanning RS, have enabled clinicians to acquire high-resolution Raman images and enabled a wide application potential for in situ functional analysis of biomolecules in living bodies, such as studies of intracellular drug pharmacokinetics, oxygen saturation of blood capillaries, and colored molecular imaging. When combined with tiny Raman tags in the cellular silent region, Raman microscopy has the capability to map the distribution of specific target small molecules in living cells and has the potential for the observation and analysis of biological functions during endoscopic examinations.
IMS is currently receiving large attention because of its accuracy and clinical potential, although its use is not yet well known to clinicians because there is still room for its further development for clinical applications. As matrix-assisted laser desorption/ionization time-of-flight (MALDI)-IMS can show the biomolecular changes in cells as well as in tissues, it can be an ideal tool for the molecular diagnosis of clinical specimens. Especially, its attractive point might be that prompt detection of premalignant lesions is possible much earlier before overt cancerous changes, that is, at an earlier stage before confirmative pathological diagnosis with endoscopic biopsy or CLE. Even in a single cell and tissue, MALDI-IMS is also a powerful tool for the detection and localization of drugs, proteins, and lipids and can be a more powerful tool for investigating the spatial distribution of biomolecules without any time-consuming extraction, purification, and separation procedures.
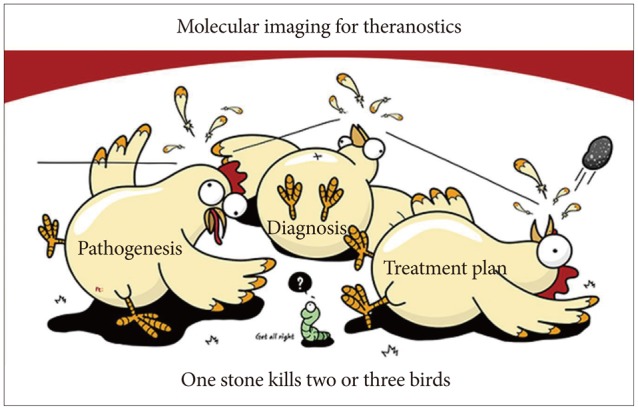
As much as the advances in these analytical technologies, the technology of nanomedicine is also a very important factor in either the analytical application or theranostics feasibility of molecular imaging. Paralleled by advances in chemistry, biology, pharmacology, nanotechnology, medicine, endoscopy, and imaging technology, several different systems have been developed in the last decade by which disease diagnosis and therapy are simultaneously performed. The so-called theranostics combines a drug and a molecular imaging agent within a single formulation, as besides for imaging applications, nanomedicine formulations have also been increasingly employed in recent years for treatment purposes simultaneously. Therefore, the strategy of "one stone to kill two or three birds" is set, comprising the accurate characterization of cellular status with a biomarker and molecular imaging as well as targeted therapy. Therefore, theranostics can be a highly suitable and ideal system for monitoring drug delivery, drug release, and drug efficacy in the era of personalized medicine, even "killing almost four birds with one stone" in the near future. With the application of nanotechnology in the biomedical field (known as nanomedicine), the creation of "nanotheranostics" (i.e., theranostic nanomedicines), integrated imaging and therapeutic functions in a single platform, and use of current molecular imaging tools such as RS all make nanotheranostics extremely appealing to personalized medicine in order to achieve the maximal benefit together with a guarantee of a high safety profile. In this review article, further indepth information about the recent advancements in molecular imaging are discussed, together with our experiences with molecular imaging in Barrett's esophagus, chronic atrophic gastritis, and inflammatory bowel disease, enabling to achieve personalization of nanomedicine-based diagnostic or therapeutic interventions in the near future under the scope of molecular imaging.
As an effort to diagnose invisible precancerous lesion in patients undergoing endoscopy, Badizadegan et al.,5 by using optical spectroscopy such as the Fast-EEM (an optical fiber clinical device that collects spectra of reflected light and fluorescence at multiple excitation wavelengths from the tissue to study small regions of tissue) and the light scattering spectroscopy (LSS) imaging system, have studied quantitative information in real time on patients undergoing routine premalignant lesion surveillance of the oral cavity, uterine cervix, and gastrointestinal (GI) tract, without removing the tissue and without the need for staining and fixation, respectively.6 As a result, the LSS imaging system provided wide-area spectroscopic images of the epithelium, depicting the nuclear size, degree of pleomorphism, degree of hyperchromasia, and amount of chromatin in the cellular level-key parameters in diagnosing precancerous lesions. Reflectance spectroscopy can probe changes in epithelial nuclei that are important findings of nuclear atypia associated with precancerous and cancerous changes.7 Fluorescence spectroscopy can assess mitochondrial fluorophores and epithelial stromal interactions by allowing investigating the decrease in collagen cross-link fluorescence that occurs in premalignant lesions, all of which are useful in detecting precancerous lesions.8
RS, an optical technique capable of identifying the chemical constituents of a sample by their unique set of molecular vibrations based on the inelastic scattering of a photon, as described in the Introduction, already has been applied and yielded successful results in the context of prostate, breast, brain, skin, and head and neck cancers; pediatric tumors; and GI cancers, differentiating cancerous from normal tissues precisely.9,10 For GI tract application, recent advancements in surface enhanced Raman scattering (SERS) contributed to an extremely high enhancement, compensating for the weak inelastic scattering effect of photons before SERS application.11,12,13 This achievement opens exciting opportunities for applications of vibrational spectroscopy in biology, as SERS is a powerful spectroscopic technique capable of detecting trace amounts of chemicals and identifying them through their unique vibrational characteristics.14 These developments in SERS have been extended further to two-photon excitation by exploiting surface-enhanced hyper-Raman scattering. These SERS nanoparticle tags provided much brighter spectroscopic information than the previous semiconductor quantum dots, raising new opportunities for multiplexed molecular diagnosis with in vivo RS.15,16 Therefore, SERS microscopy, a technique combining the advantages of biofunctionalized metal nanoparticles and RS to visualize and quantify the distribution of target molecules in both cells and tissues, might be a novel method of vibrational microspectroscopic imaging.17,18 Since the potential of SERS for widespread use will likely be realized only with the development of cheaper, simpler methods,19 SERS is now facing additional challenges such as the development of a multiplexing and high-throughput screening method.20,21 Lim et al.22 and Hossain et al.23 recently successfully programed nanostructure fabrication and single-DNA detection strategies that can open new avenues for the high-yield synthesis of optically active smart nanoparticles and reproducible nanostructure-based single-molecule detection. Zavaleta et al.24 and Palonpon et al.25 successfully showed great potential for multiplexed imaging in living subjects by using these targeted SERS probes, and offered better demonstration of multiple biomarkers associated with a specific disease, achieving advances in cell imaging techniques based on spontaneous Raman scattering and highlighting its potential for observation.26 Harada and Takamatsu,27 Kendall et al.,28 and Almond et al.29 compared and evaluated the efficacy of RS, respectively. For instance, through an objective identification in 62 patients with Barrett's esophagus in vitro, they found that RS demonstrated a sensitivity of 86% and a specificity of 88% in discriminating high-grade dysplasia and adenocarcinoma. As a real clinical application of RS endoscopy, Wang et al.30 developed a beveled fiber-optic confocal Raman probe coupled with a ball lens for enhancing the in vivo epithelial tissue Raman measurements on endoscopy, and found the great potential of applying this Raman probe for improving the in vivo diagnosis of precancerous lesions occurring in epithelial tissue on endoscopy. Taketani et al.31 applied a miniaturized Raman endoscope system to monitor the advancement of colorectal tumors in model mice. Use of a fiber opticbased RS device with SERS nanoparticles as molecular imaging contrast agents could allow endoscopists to distinguish between normal and precancerous tissues rapidly and to identify flat lesions missed with conventional methods.32 Bergholt et al.33,34 and Huang et al.35 concluded that RS is useful for monitoring intestinal-type gastric carcinogenesis to realize the early diagnosis and detection of premalignant lesions as well as early gastric cancer during clinical endoscopic examination. Our group also applied RS endoscopy to perform a proper evaluation of chronic atrophic gastritis after an appropriate treatment in the clinic, because the interobserver agreement between pathologists was lower with the updated Sydney system.36
MALDI-IMS is emerging as a powerful tool for investigating the distribution of molecules within biological systems through the direct molecular analysis of thin tissue sections,37 allowing the investigation of the molecular content of tissues within the morphological context without using any staining antibodies. As it is able to measure the distribution of hundreds of analytes at once, while being label free, this method has great potential that has been increasingly recognized in the field of tissue-based research in the last few years.38 Because tissues are analyzed intact without homogenization, the spatial relations of molecules are preserved.39 However, still, several technical refinements are prerequisites for the full exploitation of this technology, such as tissue collection, target-specific tissue pretreatment, matrix choice for efficient ionization, and matrix deposition to improve imaging resolution; moreover, rather expensive instruments and the accumulated experience of the technology are needed. Besides the discovery of unknown biomarkers against clinical disease, MALDI-IMS has the versatility of use in several fields such as medicine, agriculture, biology, pharmacology, and pathology.40 Our group has shown the application of MALDI-IMS in the diagnosis of colitis-associated cancer and Barrett's esophagus,41,42 by which specific molecular imaging of colitic cancer could be obtained without using immunohistochemical staining, as well as a high discriminating efficiency with a label-free quantification method. Setou and Kurabe43 had introduced similar works to those of our group with MALDI-IMS, with special reference to its applications in biomolecular analyses; the workflow and principle of IMS; and other related technologies.
Personalized medicine is the future dream of patient management-a treatment not based on trial and error; however, it should incorporate an integration of different fields such as chemistry, engineering, biology, technology, and medicine, based on essential modalities such as optical imaging, CT, ultrasonography, MRI imaging, PET, and PET-CT, as mentioned before, to achieve the modern "4P medicine" (i.e., predictive, preventive, precise, and personalized medicine).44 Therefore, the use of multifunctional nanoplatforms over traditional approaches might be essential to facilitate these achievements, in which spherical constructs such as liposomes, micelles, nanoemulsions, macromolecules, dendrimers, and solid nanoparticle structures are also prerequisite for fulfilling personalized molecular imaging.45 In the next decade, new molecular imaging nanostructures will be designed as multifunctional constructs to both amplify imaging signals at disease sites and deliver localized therapy. The development and clinical translation of next-generation nano-structures will be facilitated by a combination of improved clarity of in vivo imaging and intercalating biological challenge implicated in disease pathogenesis. Recent advances in optical molecular imaging can allow the identification of morphologic and biochemical changes in GI premalignant tissues much earlier before overt clinical presentation and in real-time manner. For instance, Barrett's esophagus and ulcerative colitis, both premalignant lesions of esophageal adenocarcinoma and colon adenocarcinoma, can be traced and tracked before the manifestation of overt tumors with the application of molecular imaging. These achievements enable delivering specific targeted ablation therapy of precancerous lesions in conjunction with earlier diagnosis through molecular imaging.41
In recent years, the use of nanomedicine formulations for therapeutic and diagnostic applications has increased exponentially. These so-called theranostics are able to provide valuable information on drug delivery, drug release, and drug efficacy, which can be highly useful for personalizing nano-medicine-based diagnostics as well as providing efficient therapeutic intervention.46 Theranostic agents represent a recently introduced class of imaging probes designed to offer pharmacologists and physicians a robust tool for minimally invasive in vivo visualization of drug delivery or release, as well as therapeutic monitoring.47 The expected end-point of theranostic agents is to provide a fundamental support for the optimization of innovative diagnostic and therapeutic strategies simultaneously, contributing to the realization of "personalized medicine" in high-risk or early-stage GI cancer patients. However, although a growing number of advanced anticancer nanomedicines such as Doxil, Lipoxal, and Depo-Cyte have already entered into different phases of clinical trials,48 most of these namomedicines fail to clearly differentiate diseased from normal cells, and thus require further advancement in "on-demand" imaging or sensing of target molecules with a high discriminating power. Although a higher detection of premalignant lesions than before has been achieved, further improvement is still needed for the clinical application of theranostics. Many different systems and strategies should be developed for drug targeting to pathological sites, as well as for visualizing and quantifying important pathophysiological processes in the near future. Concerning personalized medicine, in the current stage, it can be reasoned that only in patients who show high levels of target site accumulation, and who respond well to the first couple of treatment cycles, should targeted therapy be continued; for other patients, other therapeutic options should be considered. On the basis of these insights, although the potential applications of theranostic nanomedicine range from the noninvasive assessment of the biodistribution and target site of accumulation of low-molecular-weight drugs, as well as the visualization of drug distribution and drug release at the target site, to the optimization of strategies relying on triggered drug release and the prediction and real-time monitoring of therapeutic responses,49,50 the potential of nanometer-sized agents for early detection, diagnosis, and personalized treatment of diseases is still under trial.51 The hope and hype of theranostics is still under progress for future medicine.
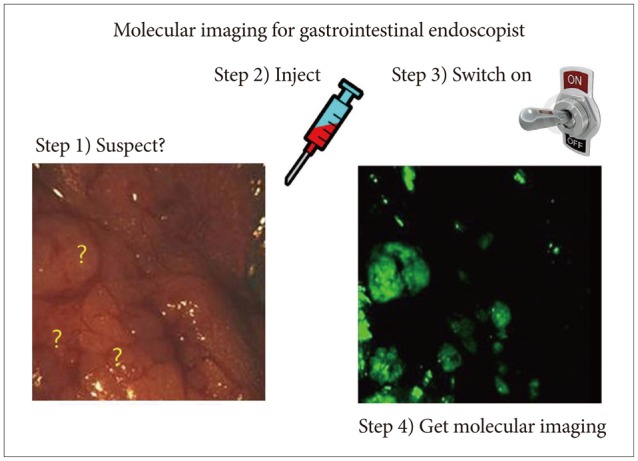
Recent advances in molecular imaging allow the identification of both morphologic and molecular changes in tissues apart from the diagnosis based on morphological changes alone with the current endoscopy techniques. For instance, GI premalignant lesions can be suspected far before the detection of stigmata or small lesions with the introduction of modern technologies such as NBI, AFI, CLE, high-resolution microendoscopy, and optical CT. Like fingerprinting technology, which is very popularly used in several areas, GI mucosa "fingerprinting" can be possible with RS endoscopy, allowing monitoring the progression of premalignant lesions, diagnosis, and follow-up after therapy. Moreover, IMS can also provide extraordinary information of GI epithelial changes and allow imaging without any contrast agent or staining application. These advanced modern technologies of molecular imaging will lead to the detection of precancerous lesions much earlier before the current high-resolution magnifying endoscopy and can predict clinical prognosis and treatment efficacy, far beyond the current advancements in optical imaging. To summarize the aforementioned findings about the hope of molecular imaging in gastroenterology, in the near future, endoscopists will finish their endoscopic examinations with two additional steps after the current endoscopy examination: (1) injection of nanoparticles according to suspicious lesions on endoscopy and (2) turning on the switch for "detecting light." These steps will conclude the endoscopy examination before the final pathology report (Fig. 1). Probably, these simple molecular imaging procedures will provide more information beyond the pathology report. The 4P medicine under tailored molecular imaging will lead to the choice of optimal targeted treatment, including theranostics (Fig. 2), and can predict prognosis in patients with GI cancers. Although it has been invented for more than five decades, RS endoscopy can be applied in the clinic as an inventory endoscopy technique, either to monitor gastric pathology or to detect pathologies that go unnoticed by current modalities. However, clinicians should spend more time in learning the principles of spectroscopy and training in the "hawk-eye" interpretation of underlying pathologies to enjoy these advancements of modern molecular imaging.
References
1. Miller JC, Thrall JH. Commission of Molecular Imaging, American College of Radiology. Clinical molecular imaging. J Am Coll Radiol. 2004; 1(1 Suppl):4–23. PMID: 17411749.

2. Rollo FD. Molecular imaging: an overview and clinical applications. Radiol Manage. 2003; 25:28–32. PMID: 12817419.
3. Li KC. From molecular imaging to systems diagnostics: time for another paradigm shift? Eur J Radiol. 2009; 70:201–204. PMID: 19261418.

4. Cho WY, Jang JY, Lee DH. Endoscopic Technology and Investigation Study Group. Recent advances in image-enhanced endoscopy. Clin Endosc. 2011; 44:65–75. PMID: 22741116.

5. Badizadegan K, Backman V, Boone CW, et al. Spectroscopic diagnosis and imaging of invisible pre-cancer. Faraday Discuss. 2004; 126:265–279. PMID: 14992412.

6. Qiu L, Turzhitsky V, Chuttani R, et al. Spectral imaging with scattered light: from early cancer detection to cell biology. IEEE J Sel Top Quantum Electron. 2012; 18:1073–1083. PMID: 23087592.

7. Gurjar RS, Backman V, Perelman LT, et al. Imaging human epithelial properties with polarized light-scattering spectroscopy. Nat Med. 2001; 7:1245–1248. PMID: 11689891.

8. Sokolov K, Follen M, Richards-Kortum R. Optical spectroscopy for detection of neoplasia. Curr Opin Chem Biol. 2002; 6:651–658. PMID: 12413550.

9. Devpura S, Barton KN, Brown SL, et al. Vision 20/20: the role of Raman spectroscopy in early stage cancer detection and feasibility for application in radiation therapy response assessment. Med Phys. 2014; 41:050901. PMID: 24784365.

10. Keating ME, Byrne HJ. Raman spectroscopy in nanomedicine: current status and future perspective. Nanomedicine (Lond). 2013; 8:1335–1351. PMID: 23914968.

11. Kneipp J, Kneipp H, Kneipp K. SERS: a single-molecule and nanoscale tool for bioanalytics. Chem Soc Rev. 2008; 37:1052–1060. PMID: 18443689.
12. Schlücker S. Surface-enhanced Raman spectroscopy: concepts and chemical applications. Angew Chem Int Ed Engl. 2014; 53:4756–4795. PMID: 24711218.

13. Zhang Y, Hong H, Cai W. Imaging with Raman spectroscopy. Curr Pharm Biotechnol. 2010; 11:654–661. PMID: 20497112.

14. Zhang Y, Hong H, Myklejord DV, Cai W. Molecular imaging with SERS-active nanoparticles. Small. 2011; 7:3261–3269. PMID: 21932216.

15. Qian XM, Nie SM. Single-molecule and single-nanoparticle SERS: from fundamental mechanisms to biomedical applications. Chem Soc Rev. 2008; 37:912–920. PMID: 18443676.

16. Han XX, Zhao B, Ozaki Y. Surface-enhanced Raman scattering for protein detection. Anal Bioanal Chem. 2009; 394:1719–1727. PMID: 19267242.

17. Schlücker S. SERS microscopy: nanoparticle probes and biomedical applications. Chemphyschem. 2009; 10:1344–1354. PMID: 19565576.

18. Jun BH, Kim G, Noh MS, et al. Surface-enhanced Raman scattering-active nanostructures and strategies for bioassays. Nanomedicine (Lond). 2011; 6:1463–1480. PMID: 22026382.

19. Betz JF, Yu WW, Cheng Y, White IM, Rubloff GW. Simple SERS substrates: powerful, portable, and full of potential. Phys Chem Chem Phys. 2014; 16:2224–2239. PMID: 24366393.

20. Abalde-Cela S, Aldeanueva-Potel P, Mateo-Mateo C, Rodriguez-Lorenzo L, Alvarez-Puebla RA, Liz-Marzán LM. Surface-enhanced Raman scattering biomedical applications of plasmonic colloidal particles. J R Soc Interface. 2010; 7(Suppl 4):S435–S450. PMID: 20462878.

21. Garai E, Sensarn S, Zavaleta CL, et al. High-sensitivity, real-time, ratiometric imaging of surface-enhanced Raman scattering nanoparticles with a clinically translatable Raman endoscope device. J Biomed Opt. 2013; 18:096008. PMID: 24008818.

22. Lim DK, Jeon KS, Kim HM, Nam JM, Suh YD. Nanogap-engineerable Raman-active nanodumbbells for single-molecule detection. Nat Mater. 2010; 9:60–67. PMID: 20010829.

23. Hossain MK, Kitahama Y, Huang GG, Han X, Ozaki Y. Surface-enhanced Raman scattering: realization of localized surface plasmon resonance using unique substrates and methods. Anal Bioanal Chem. 2009; 394:1747–1760. PMID: 19384546.

24. Zavaleta CL, Smith BR, Walton I, et al. Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy. Proc Natl Acad Sci U S A. 2009; 106:13511–13516. PMID: 19666578.

25. Palonpon AF, Sodeoka M, Fujita K. Molecular imaging of live cells by Raman microscopy. Curr Opin Chem Biol. 2013; 17:708–715. PMID: 23773582.

26. Zavaleta CL, Hartman KB, Miao Z, et al. Preclinical evaluation of Raman nanoparticle biodistribution for their potential use in clinical endoscopy imaging. Small. 2011; 7:2232–2240. PMID: 21608124.

27. Harada Y, Takamatsu T. Raman molecular imaging of cells and tissues: towards functional diagnostic imaging without labeling. Curr Pharm Biotechnol. 2013; 14:133–140. PMID: 22356110.

28. Kendall C, Stone N, Shepherd N, et al. Raman spectroscopy, a potential tool for the objective identification and classification of neoplasia in Barrett's oesophagus. J Pathol. 2003; 200:602–609. PMID: 12898596.

29. Almond LM, Hutchings J, Lloyd G, et al. Endoscopic Raman spectroscopy enables objective diagnosis of dysplasia in Barrett's esophagus. Gastrointest Endosc. 2014; 79:37–45. PMID: 23886354.
30. Wang J, Bergholt MS, Zheng W, Huang Z. Development of a beveled fiber-optic confocal Raman probe for enhancing in vivo epithelial tissue Raman measurements at endoscopy. Opt Lett. 2013; 38:2321–2323. PMID: 23811915.

31. Taketani A, Hariyani R, Ishigaki M, Andriana BB, Sato H. Raman endoscopy for the in situ investigation of advancing colorectal tumors in live model mice. Analyst. 2013; 138:4183–4190. PMID: 23762892.

32. Zavaleta CL, Garai E, Liu JT, et al. A Raman-based endoscopic strategy for multiplexed molecular imaging. Proc Natl Acad Sci U S A. 2013; 110:E2288–E2297. PMID: 23703909.

33. Bergholt MS, Zheng W, Ho KY, et al. Fiber-optic Raman spectroscopy probes gastric carcinogenesis in vivo at endoscopy. J Biophotonics. 2013; 6:49–59. PMID: 23288709.
34. Bergholt MS, Zheng W, Lin K, et al. In vivo diagnosis of gastric cancer using Raman endoscopy and ant colony optimization techniques. Int J Cancer. 2011; 128:2673–2680. PMID: 20726002.

35. Huang Z, Teh SK, Zheng W, et al. In vivo detection of epithelial neoplasia in the stomach using image-guided Raman endoscopy. Biosens Bioelectron. 2010; 26:383–389. PMID: 20729057.

36. Bergholt MS, Zheng W, Lin K, et al. Raman endoscopy for in vivo differentiation between benign and malignant ulcers in the stomach. Analyst. 2010; 135:3162–3168. PMID: 20941419.

37. Cornett DS, Reyzer ML, Chaurand P, Caprioli RM. MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat Methods. 2007; 4:828–833. PMID: 17901873.

38. Balluff B, Schöne C, Höfler H, Walch A. MALDI imaging mass spectrometry for direct tissue analysis: technological advancements and recent applications. Histochem Cell Biol. 2011; 136:227–244. PMID: 21805154.

39. Kubo A, Kajimura M, Suematsu M. Matrix-assisted laser desorption/ionization (MALDI) imaging mass spectrometry (IMS): a challenge for reliable quantitative analyses. Mass Spectrom (Tokyo). 2012; 1:A0004. PMID: 24349905.

40. Zaima N, Hayasaka T, Goto-Inoue N, Setou M. Matrix-assisted laser desorption/ionization imaging mass spectrometry. Int J Mol Sci. 2010; 11:5040–5055. PMID: 21614190.

41. Ko KH, Kwon CI, Park SH, et al. Application of matrix-assisted laser desorption/ionization time-of-flight imaging mass spectrometry (MALDI-TOF IMS) for premalignant gastrointestinal lesions. Clin Endosc. 2013; 46:611–619. PMID: 24340253.

42. Ko KH, Han NY, Kwon CI, et al. Recent advances in molecular imaging of premalignant gastrointestinal lesions and future application for early detection of barrett esophagus. Clin Endosc. 2014; 47:7–14. PMID: 24570878.

43. Setou M, Kurabe N. Mass microscopy: high-resolution imaging mass spectrometry. J Electron Microsc (Tokyo). 2011; 60:47–56. PMID: 21109523.

44. Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007; 3:1840–1854. PMID: 17943716.

45. Matsuura N, Rowlands JA. Towards new functional nanostructures for medical imaging. Med Phys. 2008; 35:4474–4487. PMID: 18975695.

46. Rizzo LY, Theek B, Storm G, Kiessling F, Lammers T. Recent progress in nanomedicine: therapeutic, diagnostic and theranostic applications. Curr Opin Biotechnol. 2013; 24:1159–1166. PMID: 23578464.

47. Terreno E, Uggeri F, Aime S. Image guided therapy: the advent of theranostic agents. J Control Release. 2012; 161:328–337. PMID: 22626940.

48. Barar J, Omidi Y. Surface modified multifunctional nanomedicines for simultaneous imaging and therapy of cancer. Bioimpacts. 2014; 4:3–14. PMID: 24790893.
49. Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic nanomedicine. Acc Chem Res. 2011; 44:1029–1038. PMID: 21545096.

50. Lammers T, Kiessling F, Hennink WE, Storm G. Nanotheranostics and image-guided drug delivery: current concepts and future directions. Mol Pharm. 2010; 7:1899–1912. PMID: 20822168.

51. Pan D, Caruthers SD, Chen J, et al. Nanomedicine strategies for molecular targets with MRI and optical imaging. Future Med Chem. 2010; 2:471–490. PMID: 20485473.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download