Abstract
Endoscopy for acute nonvariceal upper gastrointestinal bleeding plays an important role in primary diagnosis and management, particularly with respect to identification of high-risk stigmata lesions and to providing endoscopic hemostasis to reduce the risk of rebleeding and mortality. Early endoscopy, defined as endoscopy within the first 24 hours after presentation, improves patient outcome and reduces the length of hospitalization when compared with delayed endoscopy. Various endoscopic hemostatic methods are available, including injection therapy, mechanical therapy, and thermal coagulation. Either single treatment with mechanical or thermal therapy or a treatment that combines more than one type of therapy are effective and safe for peptic ulcer bleeding. Newly developed methods, such as Hemospray powder and over-the-scope clips, may provide additional options. Appropriate decisions and specific treatment are needed depending upon the conditions.
Go to : 
Acute nonvariceal upper gastrointestinal bleeding (NVUGIB) is a gastrointestinal emergency that has considerable morbidity and mortality. Peptic ulcer bleeding is the most common cause of upper gastrointestinal bleeding (UGIB), and it is responsible for about 31% to 67% of all cases, followed by erosive disease and variceal bleeding. Mallory-Weiss tears, Dieulafoy's lesions, vascular ectasia, and neoplasm comprise the remainder of the possible causes.1 The mortality rate has remained unchanged at 3.5% to 7.4% over the past several decades.2,3,4 Higher mortality is likely associated with one of the following features: hemoglobin <7.0 g/dL, American Society of Anesthesiologists class 4, age >80, renal failure, rebleeding, and failure of endoscopic treatment.5,6 Seventy percent of UGIB is recovered spontaneously without recurrence; however, 10% of patients continue to bleed and 20% experience continued or recurrent bleeding in the first 24 to 72 hours.7 Most high-risk stigmata lesions require around 72 hours to regress into a low-risk appearance after endoscopic therapy, and most rebleeding (about 80%) in high-risk patients occurs within the first 72 hours.8 Therefore, it is suggested that patients with high-risk lesions be admitted to hospital for the 72 hours of high-dose intravenous proton pump inhibitors (IV PPI) therapy after endoscopic hemostasis.8 Furthermore, IV PPI has been found to be cost-effective because it lowers the ration of patients with active bleeding lesions on endoscopic view, thus it reduced the need for further intervention.9,10,11
Go to : 
Before endoscopy, several preventive management strategies can be used to reduce hemostasis-related adverse effects, including volume replacement, restoration of hemodynamic stability, and amendment of coagulopathy. On presentation with UGIB, immediate evaluation and appropriate resuscitation should be carried out. The nasogastric tube insertion in selected patients should be considered because the findings may have prognostic value. Patients should be stratified into low-risk and high-risk groups by using prognostic scales, laboratory data, and endoscopic criteria, as well as by applying a clinical assessment. Very low-risk patients may be discharged, but all other patients should be hospitalized and categorized as low-risk or high-risk to determine treatment options.12,13
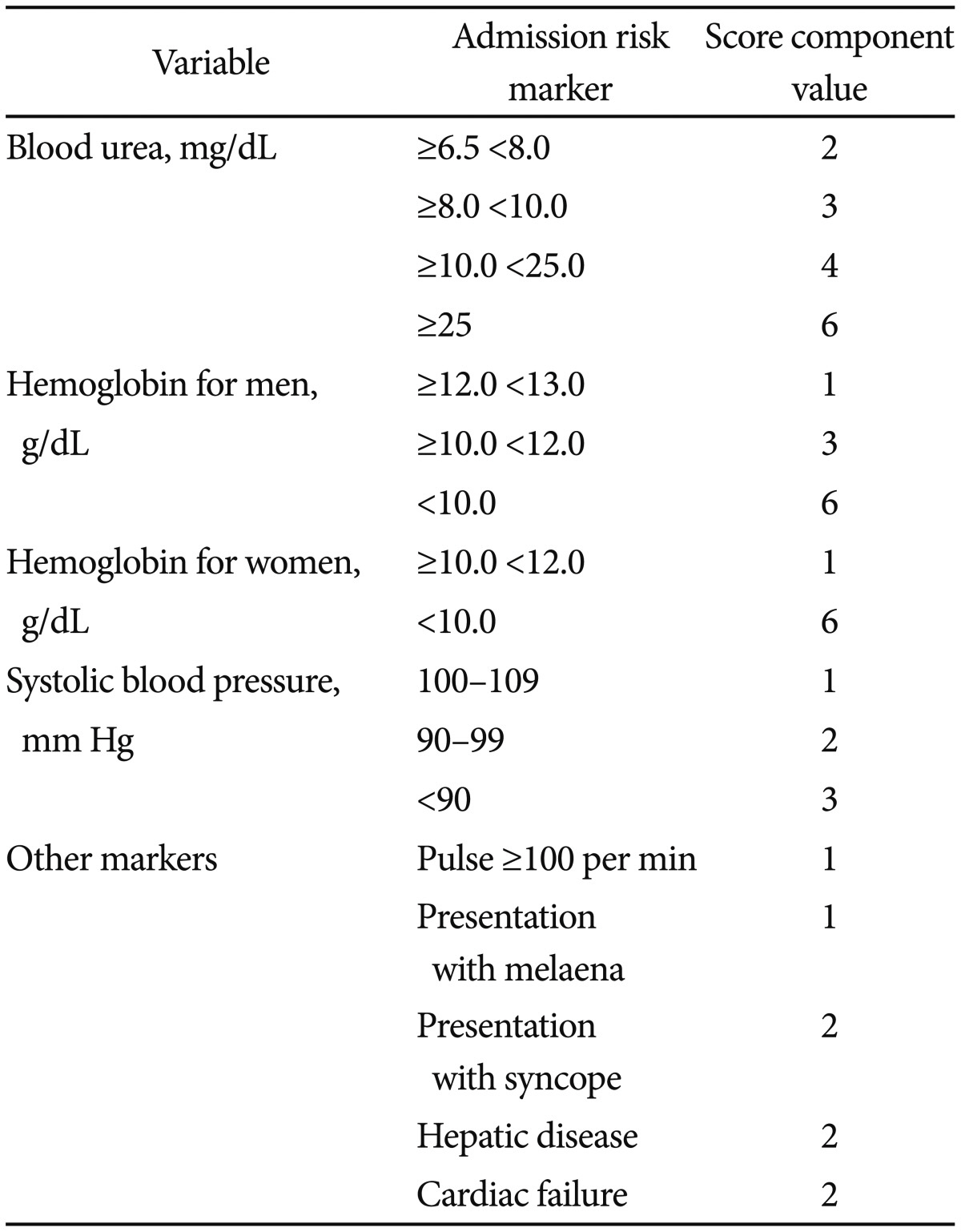
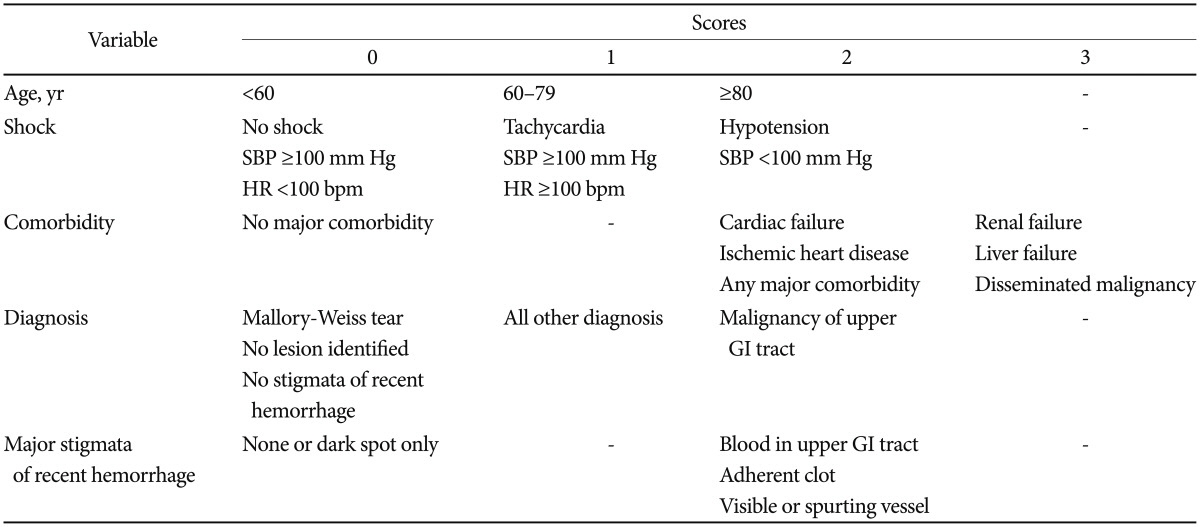
Blood transfusions should be considered for a patient with a hemoglobin level of 7.0 g/dL or less. Oral PPI therapy can be administered for low-risk patients, but high-risk patients should be treated with endoscopic and IV high-dose PPI. IV PPI therapy prior to endoscopy may be considered to downstage the endoscopic lesion and decrease the need for endoscopic intervention but it should not delay endoscopy. All patients should be considered for secondary prophylaxis, including a Helicobacter pylori test and treatment, the use of cyclooxygenase-2 antagonists as an alternative to nonsteroidal anti-inflammatory drugs (NSAIDs), and PPI for those taking low-dose aspirin.12,13 The Glasgow-Blatchford scoring system may be used at first assessment, but after endoscopy the full Rockall scoring system should be followed.14,15,16 The scores are calculated using the tables below (Tables 1, 2).
Go to : 
Endoscopy is important for the primary diagnosis and management of acute NVUGIB. Early endoscopy (within 24 hours of presentation) is recommended for most patients with acute UGIB.13 Early endoscopy is related to significant reductions in length of hospital stay, as compared to delayed endoscopy and a decreased need for surgery in elderly patients. Rapid endoscopy within 6 hours did not demonstrate advantages in the outcomes of mortality, need for surgery and transfusion requirements compared with endoscopy within 24 hours.17,18
High-risk lesions for rebleeding include actively spurting lesions (Forrest class IA), oozing blood (class IB), a nonbleeding visible vessel (class IIA), and an adherent clot (class IIB). Low-risk lesions include flat pigmented spots (Forrest class IIC) and clean-based ulcers (class III).19 An endoscopic hemostatic procedure is not required for patients with low-risk stigmata (a clean-based ulcer [III] or a flat pigmented spots [IIc]). A clot in an ulcer bed (IIB) needs to be removed with targeted irrigation, and appropriate treatment should be performed for the underlying lesion. The necessity of endoscopic therapy for ulcers with adherent clots is still debating. Endoscopic hemostatic therapy is required for patients with high-risk stigmata (active bleeding [IA, IB] or a visible vessel in an ulcer bed [IIA]).19 Epinephrine injection is not sufficient for complete hemostasis and should be used in combination with other hemostatic modality.12,13 Clips or thermocoagulation should be used in patients with high-risk lesions, either alone or in combination with other hemostatic modalities. A second-look endoscopy is generally recommended in cases when rebleeding is suspected.13,20,21 Relevant conscious sedation and appropriate use of sedative drugs such as midazolam and propofol during endoscopic hemostasis enhances the success rates and patient's satisfaction.11,22
Go to : 
Many hemostatic methods are available for effective endoscopic hemostasis. These can be categorized based upon their mechanism of action, as follows: 1) injection therapy, 2) mechanical therapy, 3) thermal coagulation, or 4) a combination of these. Several new endoscopic treatments were introduced and applied to control NVUGIB.
Injection with diluted epinephrine is widely used due to its simplicity. The mechanisms of hemostasis are local tamponade effect and vasoconstriction. It is now clear that injection with diluted epinephrine is a suboptimal treatment.8,13 An injection of diluted epinephrine should only be used to stop or slow down bleeding in order to obtain a clear view of the artery. Either hemoclipping or thermocoagulation to the artery should be followed.13,21,23 Epinephrine injection is more beneficial than medical therapy in patients with high-risk stigmata, but it is inferior to other monotherapies, such as mechanical therapy or thermal therapy, or to combination therapies that use two or more methods.13,24 Other injection therapies using sclerosant (absolute alcohol, polidocanol) or tissue adhesives (cyanoacryalate, thrombin/fibrin glue) have been used for NVUGIB.
One of the most widely usedendoscopic mechanical modalities is a hemoclip.25 Clips have been applied for hemostasis and for closing the mucosal defects that result from endoscopic mucosal resection, fistulas and perforations of GI tracts.26 That process commonly starts with the use of hemoclips, particularly where there is a clear vessel head. Effective hemoclipping is difficult if the bleeding site is in the gastric fundus, the lesser curvature of the stomach, or the posterior wall of duodenal bulb. Hemoclips need precise deployment because inadequate clipping of only the tip of the vessel can result in potentiation or initiation of vigorous bleeding. Tangential approach of hemoclips or their applications with retroflexion of a scope often fail. The deployment of hemoclips on hard or fibrotic ulcer base often difficult.27
Band ligation is another mechanical hemostatic option, and various devices have been introduced each having individual merits. The advantages of using band ligation are: easy application, accessibility to difficult sites, and short procedure time. But it has limitations for poor visual field, unavailability in fibrotic tissue, and narrow indication. Band ligation is useful for a Dieulafoy-like ulcer, angiodysplasia lesions, and Mallory-Weiss tears, and its role is the same as that of mechanical ligation of hemoclipping.7,26
Thermal endoscopic hemostasis is somewhat easier in comparison to hemocliping.27 It can be classified as either contact or noncontact. Heater probe thermocoagulation and bipolar electrocoagulation are the examples of the thermocoagulation. Contact therapies provide appositional pressure resulting in a heat-sink effect as well as tissue coagulation with contraction of the blood vessels. Among the noncontact methods, argon plasma coagulation is available at many endoscopic facilities. It is safe given the depth of penetration (<1 mm) and relatively easy to use. However, it has a limitation due to the fact that it only provides superficial coagulation, which may miss larger deeper vessels.27
Hemoclips and thermocoagulation were found to be similar in their hemostatic efficacies.28,29 Combination therapy may have an additive effect for each modality and different mechanisms of action for each technique.28,30 However, combination therapy (injection plus second injectate, thermal, or clips) was not found to be superior to hemoclips or thermal therapy alone.28,30
Go to : 
A new endoscopic application is the use of Hemospray powder (Cook Medical Inc., Winston-Salem, NC, USA). that has an ability to increase the concentration of coagulation factors, to activate platelets, and to form a mechanical plug on an injured blood vessel.31 Upon contact with blood, the powder becomes aggregated and forms a stable mechanical plug on the bleeding site. Hemospray powders were applied for peptic ulcer bleeding, cancer-associated gastrointestinal bleeding, and in patients taking antithrombotic agents. The initial reports are feasible, but there are still have a small sample size limitation. More studies should be followed to confirm the efficacy of Hemospray in the management of UGIB.27,32,33
Recent invasive endoscopic treatments, such as endoscopic submucosal dissection and natural orifice transluminal endoscopic surgery, have provided alternative approaches to surgery. An over-the-scope clip (OTSC; Ovesco Endoscopy, Tübingen, Germany) is designed for tissue approximation.34 Retrospective studies have shown the preliminary safety and feasibility of the OTSC for the treatment of UGIB and fistulae as well as for the closure of acute GI perforations. The OTSC system shows great potential for use in endoscopic treatments that require speed and simplicity.27,34,35,36,37
Go to : 
Complications during endoscopic hemostatic procedure, including aspiration pneumonia and perforation, have been minimal. A pooled analysis for all modalities has shown a complication rate of 0.5% (95% confidence interval, 0.4 to 0.8).38 Clips and epinephrine injection had the lowest rates of perforation, while the heater probe group had the highest rate.39 Other factors, such as the quality of the endoscopist's skill, unstable patient, poor sedation, poor visual field due to blood, and difficult area of reach, can affect the complications.39
Despite studious attempts with different modalities, endoscopic hemostasis therapy is sometimes unsuccessful. Factors that could predict the failure of endoscopic hemostasis include: large ulcers (2 cm in size), located at the bulbar duodenum or the lesser curvature of the stomach, active bleeding, hemodynamic instability, and the presence of comorbid illnesses.40,41,42 Angiographic embolization may be used as a rescue therapy when there is refractory bleeding to any endoscopic hemostasis. Perforation, uncontrolled bleeding, or unstable vital signs despite repeated hemostasis would be indications of emergent surgery.43
Go to : 
Early endoscopic approachment is important in the identification and management of NVUGIB, especially for patients with high-risk lesions such as active bleeding and visible vessels. Endoscopic hemostatic therapy has been considered as the pivotal treatment for NVUGIB as it has been shown to reduce rebleeding, the need for surgery, mobidity, and mortality. Many safe and effective devices are available for endoscopic hemostasis. Although epinephrine injection provides more beneficial effects than pharmacological methods, the use of clips, thermocoagulation, or a therapeutic option that combines more than one treatment approach is more effective than injection treatment alone. Promising results have recently been reported for the application of hemostasis using Hemospray powder or OTSCs. Selection of the optimal hemostatic device depends upon the characteristics of the lesion, the physician's ability, the availability of the equipment, the patient's clinical conditions, and cost. In addition, adequate H. pylori eradication, PPI therapy, and the withdrawal of NSAIDs can further reduce the rebleeding and mortality rates.1,44
Go to : 
References
1. Holster IL, Kuipers EJ. Management of acute nonvariceal upper gastrointestinal bleeding: current policies and future perspectives. World J Gastroenterol. 2012; 18:1202–1207. PMID: 22468083.

2. Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011; 60:1327–1335. PMID: 21490373.

3. Targownik LE, Nabalamba A. Trends in management and outcomes of acute nonvariceal upper gastrointestinal bleeding: 1993-2003. Clin Gastroenterol Hepatol. 2006; 4:1459–1466. PMID: 17101296.

4. Leontiadis GI, Molloy-Bland M, Moayyedi P, Howden CW. Effect of comorbidity on mortality in patients with peptic ulcer bleeding: systematic review and meta-analysis. Am J Gastroenterol. 2013; 108:331–345. PMID: 23381016.

5. Sostres C, Lanas A. Epidemiology and demographics of upper gastrointestinal bleeding: prevalence, incidence, and mortality. Gastrointest Endosc Clin N Am. 2011; 21:567–581. PMID: 21944411.

6. van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008; 22:209–224. PMID: 18346679.

7. Chung IK. How can we maximize skills for non-variceal upper gastrointestinal bleeding: injection, clipping, burning, or others? Clin Endosc. 2012; 45:230–234. PMID: 22977808.

8. Greenspoon J, Barkun A. A summary of recent recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Pol Arch Med Wewn. 2010; 120:341–346. PMID: 20864907.

9. Lau JY, Leung WK, Wu JC, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med. 2007; 356:1631–1640. PMID: 17442905.

10. Al-Sabah S, Barkun AN, Herba K, et al. Cost-effectiveness of proton-pump inhibition before endoscopy in upper gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2008; 6:418–425. PMID: 18304891.
11. Lichtenstein DR, Jagannath S, et al. Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008; 68:815–826. PMID: 18984096.

12. Greenspoon J, Barkun A, Bardou M, et al. Management of patients with nonvariceal upper gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2012; 10:234–239. PMID: 21820395.

13. Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010; 152:101–113. PMID: 20083829.

14. Stanley AJ, Dalton HR, Blatchford O, et al. Multicentre comparison of the Glasgow Blatchford and Rockall Scores in the prediction of clinical end-points after upper gastrointestinal haemorrhage. Aliment Pharmacol Ther. 2011; 34:470–475. PMID: 21707681.

15. Stanley AJ, Ashley D, Dalton HR, et al. Outpatient management of patients with low-risk upper-gastrointestinal haemorrhage: multicentre validation and prospective evaluation. Lancet. 2009; 373:42–47. PMID: 19091393.

16. Atkinson RJ, Hurlstone DP. Usefulness of prognostic indices in upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008; 22:233–242. PMID: 18346681.

17. Sarin N, Monga N, Adams PC. Time to endoscopy and outcomes in upper gastrointestinal bleeding. Can J Gastroenterol. 2009; 23:489–493. PMID: 19623332.

18. Jairath V, Kahan BC, Logan RF, et al. Outcomes following acute nonvariceal upper gastrointestinal bleeding in relation to time to endoscopy: results from a nationwide study. Endoscopy. 2012; 44:723–730. PMID: 22752889.

19. de Groot NL, van Oijen MG, Kessels K, et al. Reassessment of the predictive value of the Forrest classification for peptic ulcer rebleeding and mortality: can classification be simplified? Endoscopy. 2014; 46:46–52. PMID: 24218308.

20. El Ouali S, Barkun AN, Wyse J, et al. Is routine second-look endoscopy effective after endoscopic hemostasis in acute peptic ulcer bleeding? A meta-analysis. Gastrointest Endosc. 2012; 76:283–292. PMID: 22695209.
21. Tsoi KK, Chan HC, Chiu PW, Pau CY, Lau JY, Sung JJ. Second-look endoscopy with thermal coagulation or injections for peptic ulcer bleeding: a meta-analysis. J Gastroenterol Hepatol. 2010; 25:8–13. PMID: 20136971.

22. Tohda G, Higashi S, Sakumoto H, Sumiyoshi K, Kane T. Efficacy and safety of nurse-administered propofol sedation during emergency upper endoscopy for gastrointestinal bleeding: a prospective study. Endoscopy. 2006; 38:684–689. PMID: 16761209.

23. Calvet X, Vergara M, Brullet E, Gisbert JP, Campo R. Addition of a second endoscopic treatment following epinephrine injection improves outcome in high-risk bleeding ulcers. Gastroenterology. 2004; 126:441–450. PMID: 14762781.

24. Vergara M, Calvet X, Gisbert JP. Epinephrine injection versus epinephrine injection and a second endoscopic method in high risk bleeding ulcers. Cochrane Database Syst Rev. 2007; (2):CD005584. PMID: 17443601.

25. Anastassiades CP, Baron TH, Wong Kee Song LM. Endoscopic clipping for the management of gastrointestinal bleeding. Nat Clin Pract Gastroenterol Hepatol. 2008; 5:559–568. PMID: 18711412.

26. Technology Assessment Committee. Chuttani R, Barkun A, et al. Endoscopic clip application devices. Gastrointest Endosc. 2006; 63:746–750. PMID: 16650531.

27. Leung Ki EL, Lau JY. New endoscopic hemostasis methods. Clin Endosc. 2012; 45:224–229. PMID: 22977807.

28. Barkun AN, Martel M, Toubouti Y, Rahme E, Bardou M. Endoscopic hemostasis in peptic ulcer bleeding for patients with high-risk lesions: a series of meta-analyses. Gastrointest Endosc. 2009; 69:786–799. PMID: 19152905.

29. Sung JJ, Tsoi KK, Lai LH, Wu JC, Lau JY. Endoscopic clipping versus injection and thermo-coagulation in the treatment of non-variceal upper gastrointestinal bleeding: a meta-analysis. Gut. 2007; 56:1364–1373. PMID: 17566018.

30. Marmo R, Rotondano G, Piscopo R, Bianco MA, D'Angella R, Cipolletta L. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol. 2007; 102:279–289. PMID: 17311650.

31. Giday SA. Preliminary data on the nanopowder hemostatic agent TC-325 to control gastrointestinal bleeding. Gastroenterol Hepatol (N Y). 2011; 7:620–622. PMID: 22299002.
32. Babiuc RD, Purcarea M, Sadagurschi R, Negreanu L. Use of Hemospray in the treatment of patients with acute UGIB: short review. J Med Life. 2013; 6:117–119. PMID: 23904868.
33. Holster IL, Kuipers EJ, Tjwa ET. Hemospray in the treatment of upper gastrointestinal hemorrhage in patients on antithrombotic therapy. Endoscopy. 2013; 45:63–66. PMID: 23208778.

34. Chan SM, Chiu PW, Teoh AY, Lau JY. Use of the over-the-scope clip for treatment of refractory upper gastrointestinal bleeding: a case series. Endoscopy. 2014; 46:428–431. PMID: 24505017.

35. Manta R, Galloro G, Mangiavillano B, et al. Over-the-scope clip (OTSC) represents an effective endoscopic treatment for acute GI bleeding after failure of conventional techniques. Surg Endosc. 2013; 27:3162–3164. PMID: 23436101.

36. Kato M, Jung Y, Gromski MA, Chuttani R, Matthes K. Prospective, randomized comparison of 3 different hemoclips for the treatment of acute upper GI hemorrhage in an established experimental setting. Gastrointest Endosc. 2012; 75:3–10. PMID: 22196807.

37. Nishiyama N, Mori H, Kobara H, et al. Efficacy and safety of over-the-scope clip: including complications after endoscopic submucosal dissection. World J Gastroenterol. 2013; 19:2752–2760. PMID: 23687412.

38. Laine L, McQuaid KR. Endoscopic therapy for bleeding ulcers: an evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol. 2009; 7:33–47. PMID: 18986845.

39. Peter S, Wilcox M. Endoscopic therapy for peptic ulcer bleeding. In : Mönkemüller K, Wilcox CM, Muñoz-Navas M, editors. Frontiers of Gastrointestinal Research. Basel: Karger;2009. p. 37–54.
40. Maggio D, Barkun AN, Martel M, Elouali S, Gralnek IM. Reason Investigators. Predictors of early rebleeding after endoscopic therapy in patients with nonvariceal upper gastrointestinal bleeding secondary to high-risk lesions. Can J Gastroenterol. 2013; 27:454–458. PMID: 23936874.

41. Marmo R, Koch M, Cipolletta L, et al. Predicting mortality in patients with in-hospital nonvariceal upper GI bleeding: a prospective, multicenter database study. Gastrointest Endosc. 2014; 79:741–749.e1. PMID: 24219820.

42. Rotondano G, Cipolletta L, Koch M, et al. Predictors of favourable outcome in non-variceal upper gastrointestinal bleeding: implications for early discharge? Dig Liver Dis. 2014; 46:231–236. PMID: 24361122.
43. Wong SK, Yu LM, Lau JY, et al. Prediction of therapeutic failure after adrenaline injection plus heater probe treatment in patients with bleeding peptic ulcer. Gut. 2002; 50:322–325. PMID: 11839708.

44. Kim SY, Hyun JJ, Jung SW, Lee SW. Management of non-variceal upper gastrointestinal bleeding. Clin Endosc. 2012; 45:220–223. PMID: 22977806.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download