INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) is an endoscopic technique performed to diagnose and treat biliary and pancreatic diseases. Complication after ERCP occurs in approximately 5% to 10% of cases, and common complications include pancreatitis, bleeding, infection, and perforation.
1 Patients with myeloproliferative disorders have a significantly increased risk of thrombohemorrhagic complications caused by clonal expansion of an odd hematopoietic stem cell.
2 Melato et al.
3 reported a patient with an intramural gastric hematoma and hemoperitoneum caused by idiopathic myelofibrosis. Recently, we encountered a case of a 77-year-old man who developed acute abdominal pain and high fever after removal of a common bile duct (CBD) stone. Clinical, abdominal computed tomography (CT), and endoscopic findings were compatible with acute duodenal ischemia and periampullary intramural hematoma. Herein, we report a case of acute duodenal ischemia and periampullary intramural hematoma that developed after an uneventful ERCP in a patient with primary myelofibrosis (PMF).
Go to :

CASE REPORT
A 77-year-old man presented with epigastric pain that began about 1 month ago. He underwent upper endoscopy at a local clinic because the pain was not relieved by a proton pump inhibitor, and also lost 3 kg of weight during the last month. However, upper endoscopy only revealed erythematous gastritis. For further evaluation, abdominal CT was performed, which showed calcified stones in the gallbladder and CBD, peripheral intrahepatic duct dilatation, and splenomegaly. He was then referred to our hospital for further management.
The patient has been taking antihypertensive medication and clopidogrel after developing a cerebral infarction 10 years previously. He was also diagnosed with gout approximately 5 years previously. On admission, his vital signs were stable but he complained of epigastric discomfort. Physical examination was unremarkable except for mild epigastric tenderness. His laboratory test results showed marked leukocytosis and thrombocytosis with a normal hemoglobin level: white blood cell count, 28,950/µL (neutrophils, 86.1%); platelet count, 537,000/µL; and hemoglobin, 13.3 g/dL. Except for a slightly prolonged activated partial thromboplastin time of 41.7 seconds (reference range, 25.3 to 41.1), the prothrombin time (11.8 seconds; international normalized ratio, 1.04) and bleeding time (109 seconds) were within normal limits. Lactate dehydrogenase (LDH) level was also increased to 1,343 IU/L. Other serologic test results were as follows: protein, 6.6 g/dL; albumin, 4.1 g/dL; total bilirubin, 0.68 mg/dL; direct bilirubin, 0.11 mg/dL; aspartate transaminase, 22 U/L; alanine transaminase, 16 U/L; alkaline phosphatase, 94 U/L; γ-glutamyl transferase, 54 U/L; amylase, 147 U/L; lipase, 14.7 U/L; blood urea nitrogen, 23.8 mg/dL; creatinine, 1.19 mg/dL; and uric acid, 9.3 mg/dL. Since biliary pain was suspected, ERCP was performed to remove the CBD stone. After endoscopic sphincterotomy (EST), a black pigment stone was successfully removed using a basket, without immediate complications, and the CBD was cleared several times with balloon sweeps (
Fig. 1). Although the patient had stopped taking clopidogrel for only 5 days at the time of ERCP, no bleeding was observed throughout the procedure. However, approximately 12 hours after ERCP, he complained of diffuse acute abdominal pain and his body temperature increased to 39.3℃. Physical examination also revealed diffuse abdominal tenderness and the white blood cell count increased to 51,430/µL (neutrophils, 88.5%). To investigate the cause of diffuse acute abdominal pain, abdominal CT was performed, which revealed severe edematous wall thickening of the duodenum and focal localized low attenuated intramural hematoma at the periampullary area, approximately 3.1×2.7 cm in diameter (
Fig. 2A, B). On endoscopy, the mucosa was diffusely edematous throughout the duodenum with linear ulcerations, and a large bulging congestive lesion was noted on the distal side of the ampulla of Vater (
Fig. 3A). To prevent bile duct obstruction by surrounding severe mucosal edema, endoscopic nasobiliary drainage tube was carefully inserted. The fever subsided and abdominal pain ameliorated after the procedure. The white blood cell count also gradually decreased to 18,590/µL. When the patient's general condition improved with fasting and administration of systemic antibiotics, laparoscopic cholecystectomy was performed to remove the gallbladder stones. Follow-up upper endoscopy performed several days after the operation showed marked improvement of the duodenal edema and intramural hematoma (
Fig. 3B). Follow-up abdominal CT performed 2 days later also showed that the edematous wall thickening of the duodenum and intramural hematoma had almost resolved (
Fig. 2C).
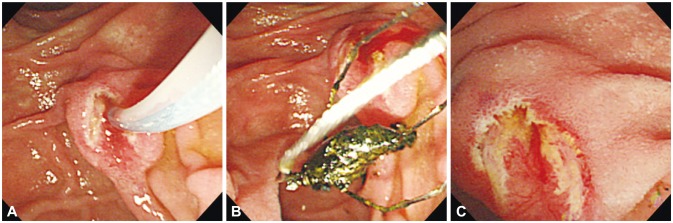 | Fig. 1(A) No bleeding occurred after endoscopic sphincterotomy. (B) A black pigment stone is successfully removed using a basket and (C) immediate complications are not observed after the procedure. 
|
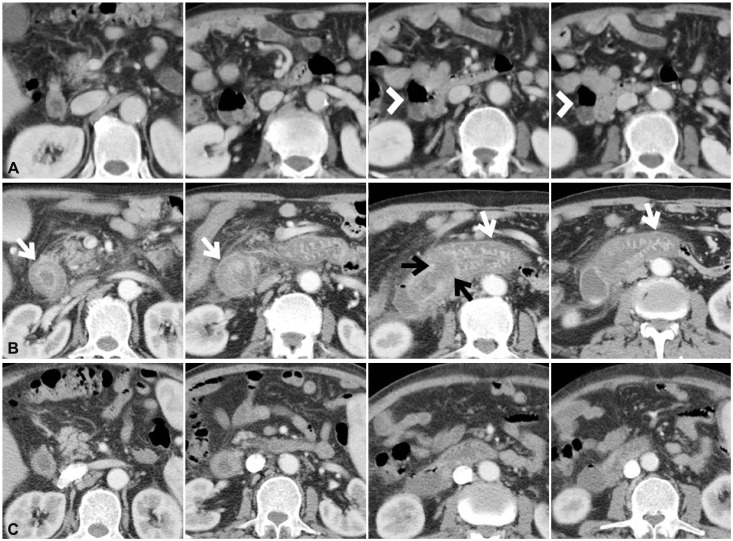 | Fig. 2(A) Initial abdomen computed tomography (CT) reveals a large duodenal diverticulum (arrowheads) on the lateral wall of duodenum second portion. (B) Abdominal CT performed the next day after endoscopic retrograde pancreaticoduodenography (ERCP) shows severe edematous wall thickening of the duodenum (white arrows). Localized focal low-attenuated lesion suggesting hematoma is also observed at the periampullary area (black arrows). (C) Follow-up abdominal CT performed 11 days after ERCP demonstrates considerably improved edematous wall thickening of the duodenum and resolved focal low-attenuated lesion at the periampullary area. 
|
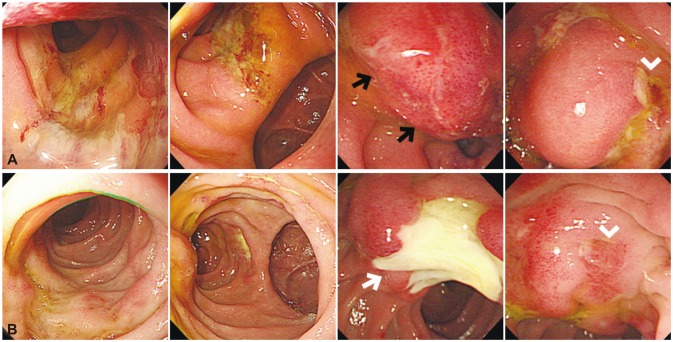 | Fig. 3(A) Endoscopic examination performed the day after endoscopic retrograde cholangiopancreatography (ERCP) demonstrates diffusely edematous duodenal mucosa with linear ulcerations and a large bulging lesion suggestive of hematoma (black arrows) distal to the ampulla of Vater (arrowhead). (B) Follow-up endoscopy performed 8 days after ERCP shows much improved duodenal wall edema with a large ulcer where the hematoma had been (white arrow). 
|
Although the patient was clinically stable and asymptomatic after the operation and the duodenal edema and intramural hematoma have resolved, the white blood cell count remained elevated at >20,000/µL. Peripheral blood smear showed hypersegmented neutrophils and teardrop cells. Therefore, myeloproliferative disease was suspected and bone marrow biopsy was performed. The bone marrow biopsy results showed hypercellular marrow with high levels of granulocytic, megakaryocytic elements, and diffuse coarse myelofibrosis that was consistent with PMF. Serologic test result for JAK2 V617F mutation was also positive. The patient was discharged afterwards without further complications and is currently being followed-up at the outpatient clinic.
Go to :

DISCUSSION
PMF is a chronic myeloproliferative disorder characterized by clonal proliferation of hematopoietic stem cells with variable hematopoietic efficiency and morphologic maturity.
4 The clinical manifestations of PMF include anemia, cachexia, hepatosplenomegaly, signs of hypermetabolic state (e.g., fever, night sweat), pruritis, portal hypertension, splenic infarction, thrombosis, and bleeding.
5 Diagnosis is made on the basis of a peripheral blood smear and clinical findings. PMF should be considered in a patient presenting with splenomegaly accompanied by unexplained anemia, elevated LDH level, leukoerythroblastosis, and teardrop red cells in the peripheral blood smear.
4,
6 The present patient had splenomegaly, elevated LDH level, and teardrop red cells in the peripheral blood smear. On the basis of these findings, the initial clinical impression included hematopoietic disorders such as PMF.
Patients with myeloproliferative disorders have a significantly increased risk of thrombohemorrhagic complications.
2 The incidence of thrombotic complication in PMF is estimated to be 2.23 events per 100 persons per year.
7 The presence of JAK2 V617F mutation, age >60 years, and leukocytosis (white blood cell count, >15,000/µL) have been reported to be significantly associated with the occurrence of thrombosis.
7 The patient in our case had all the aforementioned risk factors, and thrombosis could have been induced by the electrocautery current delivered during EST and the mechanical force exerted during CBD stone removal. Owing to the welldeveloped collateral vessels in the duodenum, thrombosis did not result in infarction but led to transient acute mesenteric ischemia, which was evidenced by the presence of sudden abdominal pain, marked leukocytosis, duodenal edema, and linear ulcerations seen endoscopically. Electrocautery current or mechanical force can cause thrombosis during ERCP. Therefore, caution through gentle handling during the procedure is needed when performing ERCP, especially in patients with thrombogenic diseases such as PMF. In patients with a history of thrombosis, the incidence of recurrent thrombotic events is known to be approximately 9%.
7 Therefore, low dose aspirin prophylaxis is recommend for patients with a history of thrombosis or a platelet count >500,000/µL.
8
PMF induces severe platelet morphofunctional abnormality with resultant hemorrhagic complications, including gastrointestinal bleeding.
9,
10 The incidence of bleeding complication in PMF is estimated to be 1.39 events per 100 persons per year.
11 Risk factors for bleeding include low dose aspirin use, leukocytosis (white blood cell count, >11,000/µL), and previous bleeding.
11 Since the duodenum is located in a fixed retroperitoneal space with rich submucosal blood supply, disruption of intramural blood vessels by shearing force applied during biopsy seems to be a predisposing factor for development of intramural hematoma.
12,
13 Biopsy was not performed in our case; however, mechanical force had to be exerted to remove the CBD stone using a basket during ERCP. Similar to the situation when taking biopsy samples, the shearing force applied during the stone extraction is likely to have led to the formation of intramural hematoma in the present case. Furthermore, current guidelines recommend withholding clopidogrel for 7 to 10 days before high-risk procedures such as EST.
14 In our patient, clopidogrel was stopped for only 5 days. Therefore, not having discontinued clopidogrel for a sufficient period might also have contributed to the development of intramural hematoma in our case.
Patients with acute mesenteric ischemia are treated to restore blood flow as soon as possible. Initial treatments include stabilization and resuscitation of the patient; however, in complicated cases such as those involving intestinal infarction or perforation, surgical treatment should not be delayed.
15 Patients with intramural hematoma respond well to conservative management consisting of parenteral nutrition, fluid and electrolyte replacement, and nasogastric suction.
16 The patient in our case also showed clinical improvement with conservative management. However, advanced treatment modality such as endoscopic incision and drainage or laparoscopic surgery should be considered when perforation or infarction is suspected, biliary or bowel obstruction occurs due to hematoma, and no clinical improvement is noted with conservative management.
17
In conclusion, acute duodenal ischemia and periampullary hematoma are rare complications after ERCP. However, they should always be considered as differential diagnoses in patients with thrombohemorrhagic disorders and if some kind of shearing force had been applied during the procedure. Awareness of findings such as splenomegaly and abnormal blood findings should be ensured, as they might suggest thrombohemorrhagic disorders such as PMF. In addition, physicians should be attentive to the signs and symptoms of intestinal ischemia and/or infarction especially in patients with PMF.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download