Abstract
Midazolam is a type of anesthetic agent frequently used for conscious sedation during a variety of medical procedures. Anaphylactic reactions to midazolam are rarely reported. However, we observed a case of midazolam hypersensitivity in which emergency measures were required to ensure patient recovery after administration of midazolam as a sedative. The occurrence of the anaphylactic reaction to midazolam was confirmed by elevated serum tryptase levels. The current case report presents a discussion of our findings.
Midazolam is a short-acting imidazobenzodiazepine depressant. In Korea, midazolam is classified in the same group of hypnotic sedatives that includes the anesthetic propofol. Midazolam is commonly used as a sedative for esophagogastroduodenoscopy (EGD) procedures, as well as for outpatient procedures that require anesthesia. Some common adverse effects of midazolam include nausea, vomiting, rhinorrhea, skin eruption, and dizziness, but the effects are almost always mild.1 In particular, drug dependency, which was a frequently reported adverse effect of propofol, is relatively rare in midazolam. Furthermore, flumazenil is used as an antidote for midazolam, and thus the drug is accepted as relatively safe.2 However, caution should still be taken when using midazolam, because there have been a limited number of reports on adverse reactions to the drug. One case of an adverse reaction has been reported in Korea,3 and three additional cases have been reported worldwide.4,5,6 In the current report, we present a case of midazolam-related anaphylactic shock that was experienced during an EGD procedure performed in a routine medical exam.
A 53-year-old woman, weighing 60 kg and with a body mass index of 23.4 kg/m2, was scheduled for an EGD during her routine medical exam. Ten years prior, she had visited the emergency medical center for allergic urticaria of unknown cause. The patient had no previous history of prescription use, smoking, or drinking. Further, no significant abnormalities were noted on her complete blood count (CBC) or blood chemistry analyses, except cholesterol and low density lipoprotein levels of 245 and 151 mg/dL, respectively. The patient's peripheral blood eosinophil count was 188 mm3 (normal range, 0 to 1,000).
The patient was fasted for ten hours, after which an intravenous cannula was secured in a peripheral vein, and a normal saline infusion was started at a rate of 40 mL/hr. Butylscopolamine (20 mg), an anticholinergic agent, was injected intravenously. After laryngo-tracheal application of 10% lidocaine spray, the patient's blood pressure, pulse, and oxygen saturation (SpO2) were monitored while in the left lateral decubitus position. Prior to anesthesia, the patient's blood pressure was 116/74 mm Hg, while her heart rate was 74 beats per minute, respiratory rate was 19 breaths per minute, and oxygen saturation was 99%. Midazolam (5 mg) was administered intravenously. Within 4 minutes after midazolam injection, the patient's pulse could not be palpated from her peripheral artery, and her SpO2 dropped 75%, after which she became cyanotic. The patient was immediately given 2 L of 100% oxygen using a nasal prong device, and 0.3 mg of flumazenil was injected intravenously. Within 5 minutes after midazolam injection, the patient showed decreased mentality, and nonspontaneous respiration. A second intravenous injection of flumazenil (0.2 mg) was administered to the patient to support her respiration, and a bag valve mask and cardiopulmonary resuscitation were used. The patient's blood pressure and peripheral pulse could not be assessed, but pupil size and pupil reflex were normal. At that time, sinus tachycardia was observed by electrocardiography (ECG) (Fig. 1). No wheezing or stridor was detected by auscultation. When tracheal intubation was performed, there was no secretion from the oral cavity or airway. The patient's SpO2 levels increased to greater than 90%, but her blood pressure remained below normal. Norepinephrine (8 µg/min) and epinephrine (1 mg) were administered intravenously. Cardiac massage was performed for 6 minutes. After norepinephrine and epinephrine administration, the patient's peripheral pulse rate was measured at 130 beats per minute, and the patient moved. The patient's blood pressure increased to 90/60 mm Hg and her respiratory rate was 24 breaths per minute. The tracheal tube was removed, and 5 L/min of oxygen was administered through a facial mask. The patient was then transferred to the intensive care unit (ICU). After rapid administration of 300 mL of normal saline, the infusion speed was adjusted to 140 mL/hr, and the patient was continually monitored. The patient developed a rash all over her body immediately. Dexamethasone (5 mg) and an antihistamine agent (3 mg) were subsequently administered via intravenous injection. The results of arterial blood gas analysis (ABGA) revealed a pH of 7.341, a blood carbon dioxide partial pressure (PaCO2) of 27.6%, a blood oxygen partial pressure (PaO2) of 97%, bicarbonate levels of 15 mmoL, and an SpO2 of 97%. No abnormal findings resulted from chemical analyses, except a lactate dehydrogenase level of 370 IU/L.
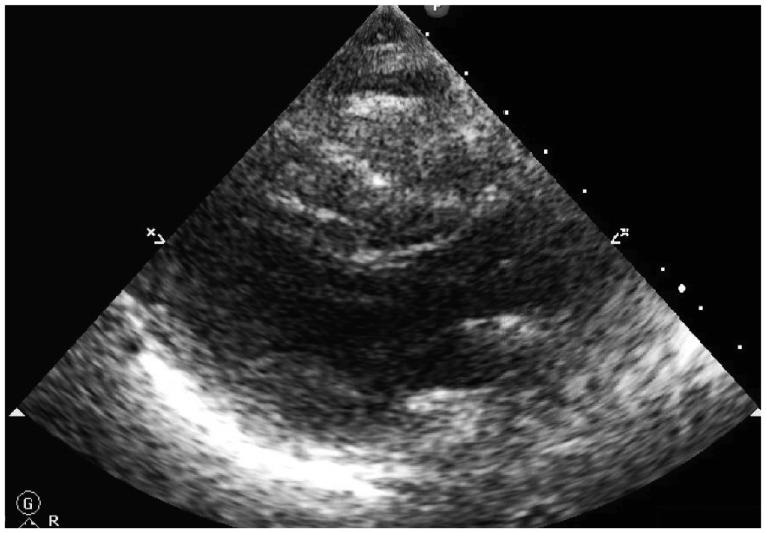
Absent stridor or wheezing on auscultation ruled out airway involvement due to the sedative effect of midazolam. There were no abnormal findings on a chest computed tomography (CT) scan performed the day following the event (Fig. 2). Among examinations performed after recovery from cardiac arrest, nonspecific findings with normal wall motion were detected by echocardiography, excepting depletion of the right ventricular volume (Fig. 3). Hematologic parameters including creatine kinase-MB and troponin I were also within the normal ranges, and thus cardiac arrest as a possible cause was also ruled out.
For one day, ABGA was repeated, and norepinephrine levels were reduced while the patient was in the ICU. The patient complained of general weakness. She was transferred to the general ward and stayed one additional day without exhibiting any abnormalities. After being discharged, the patient visited the hospital two times, but had no complaints or abnormal findings in her CBC or blood chemistry analyses. However, her blood tryptase level increased slightly during cardiac arrest to 14.8 µg/L (normal range, 1.9 to 13.5).
Midazolam is a relatively safe, short-acting imidazobenzodiazepine drug that does not have any active metabolites.7 However, several adverse effects following the use of midazolam have been reported, including respiratory suppression, bronchospasm,8 tonic-clonic seizure,9 urticaria,10 and cardiac arrhythmias.11
In particular, anaphylaxis resulting from midazolam use may occur, and is a worldwide concern that requires attention.
In a case that occurred in India last year, rash and shock resulted within 2 minutes of the intravenous administration of midazolam, in the absence of wheezing or stridor detected by auscultation. In accord with our case, all symptoms improved after the injection of epinephrine, and the administration of 100 mg of Solu-Cortef (Pharmacia Limited, Buckinghamshire, United Kingdom) and the antihistamine Plakon.4
A skin prick test, intradermal skin test, and serum tryptase assay could be used to identify the risk of anaphylaxis.7 Skin prick tests and intradermal skin tests can be performed easily, but offer limited diagnostic sensitivity and specificity.12 Serum tryptase level measurements could also be used as an indicator of anaphylaxis, as elevated serum tryptase could serve as a marker of systemic mast cell activation, through which anaphylactic reactions occur. In particular, β-tryptase is detectable in serum, with a total to β-tryptase ratio of 10 or less indicating systemic anaphylaxis.13 Elevation of tryptase in serum may indicate systemic anaphylaxis, but negative results cannot be ruled out completely.12 According to a previous study, a tryptase test showed a sensitivity of 64%, a specificity of 89.3%, a positive predictive value of 92.6%, and a negative predictive value of 54.3%.12
However, the normal range of serum tryptase is controversial. According to one study, the normal range of serum tryptase is 1 to 15 ng/mL,13 while in a separate study, the cutoff value was 25 ng/mL.12
In the current case report, the results of the tryptase test performed at the onset of symptoms were higher than the normal range, so we assumed the occurrence of anaphylaxis. To confirm, we considered performing a skin prick test to evaluate the patient's reaction to midazolam once the patient's condition was stable. However, the test was considered a high risk, and we could not obtain patient consent, so the skin prick test was not performed.
Occurrence of midazolam-associated anaphylaxis is relatively rare, so research regarding the risk factors is lacking. However, if a patient has a history of allergic urticaria, the patient requires special attention, despite the fact that a clear correlation between allergic urticaria and midazolam-associated anaphylaxis has not been established. Therefore, it is important to obtain a thorough history of allergies, and each endoscopic examination unit should be prepared to handle any accident or emergency that may occur. When symptoms are noted, emergency treatment and a serum tryptase assay are recommended as a means to evaluate the causative symptoms.
References
1. Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: pharmacology and uses. Anesthesiology. 1985; 62:310–324. PMID: 3156545.
2. Breheny FX. Reversal of midazolam sedation with flumazenil. Crit Care Med. 1992; 20:736–739. PMID: 1597024.

3. Hwang JY, Jeon YT, Na HS, Lee JH, Choi SJ, Jung SH. Midazolam hypersensitivity during the transportation to theater: a case report. Korean J Anesthesiol. 2010; 59(Suppl):S1–S2. PMID: 21286414.

4. Shrivastava S. An experience with midazolam anaphylactoid reaction. J Anesth. 2012; 26:642–643. PMID: 22467090.

5. Fujita Y, Ishikawa H, Yokota K. Anaphylactoid reaction to midazolam. Anesth Analg. 1994; 79:811–812. PMID: 7943804.

6. George C, Williams A. Anaphylaxis with midazolam: our experience. Indian J Anaesth. 2011; 55:630–631. PMID: 22223916.
7. Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003; 97:1381–1395. PMID: 14570656.

8. Davis DP, Hamilton RS, Webster TH. Reversal of midazolam-induced laryngospasm with flumazenil. Ann Emerg Med. 1998; 32:263–265. PMID: 9701315.

9. Robb ND. Epileptic fits under intravenous midazolam sedation. Br Dent J. 1996; 181:178–179. PMID: 8854426.

10. Yates A, Russell D. A reaction to midazolam: a topical solution. Anaesthesia. 1989; 44:449. PMID: 2742113.

11. Rodrigo CR, Rosenquist JB, Cheng CH. Cardiac dysrhythmias with midazolam sedation. Anesth Prog. 1990; 37:20–23. PMID: 2077981.
12. Mertes PM, Laxenaire MC, Alla F. Groupe d'Etudes des Réactions Anaphylactoïdes Peranesthésiques. Anaphylactic and anaphylactoid reactions occurring during anesthesia in France in 1999-2000. Anesthesiology. 2003; 99:536–545. PMID: 12960536.

13. Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006; 26:451–463. PMID: 16931288.

Fig. 1
Electrocardiography. (A) A normal sinus rhythm was confirmed prior to esophagogastroduodenoscopy. (B) A sinus tachycardia pattern was observed during anaphylactic shock.
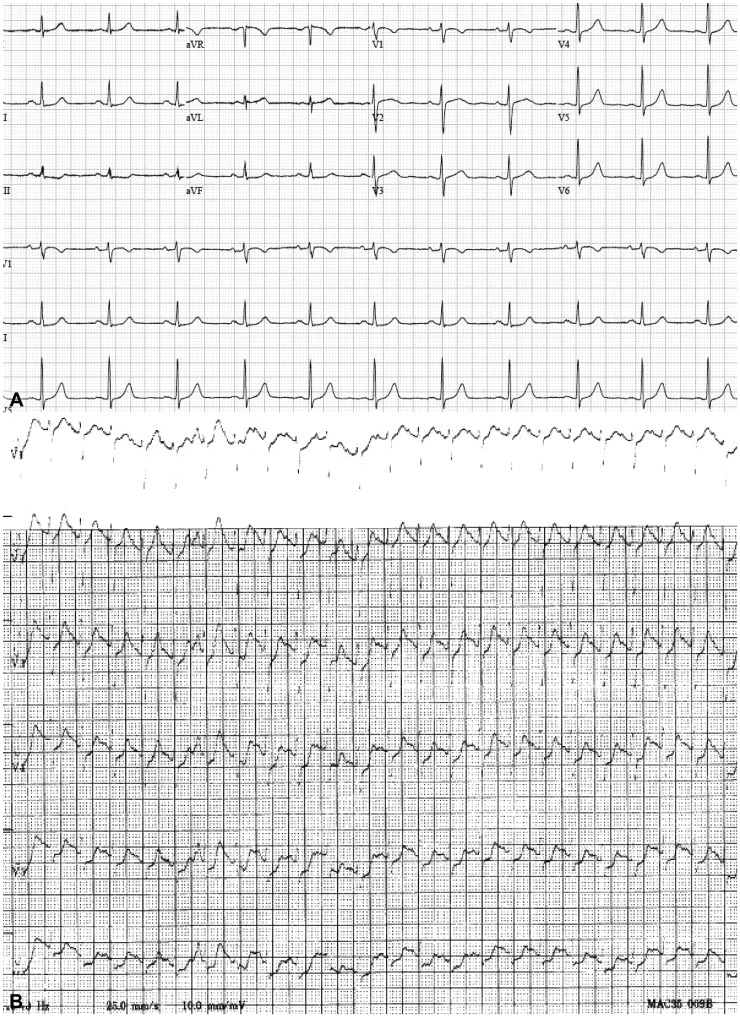
Fig. 2
Chest X-ray image finding and chest computed tomography (CT) findings. (A) No abnormal findings were noted on the chest X-ray anteroposterior image taken during the event. (B) Pleural effusion and mediastinal lymphadenopathy in both lungs were not observed in the mediastinal setting of chest CT. (C) No active lesion was observed in either lung in the lung parenchymal setting of chest CT.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download