Abstract
Background/Aims
Although postpolypectomy fever (PPF) without colon perforation or hemorrhage is rare, its incidence and risk factors have not been investigated. The objective of this study was to analyze the incidence and risk factors for PPF among inpatients.
Methods
Seven patients with PPF were matched with 70 patients without PPF from a total of 3,444 patients who underwent colonoscopic polypectomy. The PPF incidence during index hospitalization after colonoscopy was calculated, and univariate and multivariate analyses were performed to calculate the adjusted odds ratios (ORs) for risk factors.
Results
PPF without bleeding or perforation in the colon occurred in seven patients (0.2%). The median age was 58 years for cases and 61 years for controls. The median interval from polypectomy to occurrence of fever was 7 hours, and the median duration of fever was 9 hours. Polyp size >2 cm (adjusted OR, 1.08; 95% confidence interval [CI], 1.01 to 1.15; p=0.02) and hypertension (adjusted OR, 14.40; 95% CI, 1.23 to 180.87; p=0.03) were associated with a significantly increased risk of PPF. PPF increased the length of hospitalization.
The detection and removal of colorectal polyps by colonoscopic polypectomy reduces the incidence of colorectal cancer.1 Endoscopic polypectomy, however, is associated with adverse events, including bleeding, perforation, postpolypectomy electrocoagulation syndrome, and gas explosion.2 Large studies report the rates of colon perforation and hemorrhage after colonoscopic polypectomy to be ≤0.3% and 0.3% to 0.6%, respectively.3
The incidence of postpolypectomy electrocoagulation syndrome ranges from 0.07% to 1.0% in patients undergoing polypectomy. Less is known, however, about the risk factors and clinical course in postpolypectomy electrocoagulation syndrome, also called postpolypectomy syndrome, a condition characterized by the development of abdominal pain, fever, leukocytosis, and peritoneal inflammation without frank perforation of the colon.4,5,6
In real-world practice, we have often encountered patients who, after colonoscopic polypectomy, experience new-onset fever without peritoneal signs or definitive fever foci. It remains unclear, however, whether this condition, named postpolypectomy fever (PPF), is the same as postpolypectomy syndrome, as there is no clear definition of PPF.4,5
Although the rates of PPF are very low, it is difficult for clinicians to determine whether this fever represents a local or metastatic infection or is a transient event. This leads to an increase in the length of hospital stay and, consequently, in medical costs.
The purpose of the present study was to investigate the incidence of PPF and identify the risk factors associated with the development of PPF and its outcomes. This study is distinguished by the inclusion of a definitive documentation of fever (based on body temperature) after excluding other possible fever foci in patients who had undergone polypectomy.
The medical records of 5,411 patients who underwent colonoscopic polypectomy at Asan Medical Center, Seoul, Korea, between January 2005 and December 2011 were retrospectively reviewed. Of these, 1,967 patients underwent colonoscopic polypectomy in the outpatient clinic and were excluded owing to the lack of documentation of PPF. PPF was defined as follows: 1) no fever at admission; 2) no symptoms or signs of infection before polypectomy; 3) development of fever with a body temperature of >37.2℃ (98.9℉) after polypectomy during the index hospitalization period; and 4) no evidence of other explainable fever foci. Index hospitalization is defined as the first admission for polypectomy. Patients with colon perforation, hemorrhage, or symptoms or signs of infection associated with conditions other than colonoscopic polypectomy were also excluded. To identify risk factors for PPF, 10 patients without fever were randomly selected for each patient with PPF. Controls were matched according to the calendar day on which polypectomy was performed. The medical records for all cases and controls were reviewed. We decided to perform a nested case-control study because our aim was to identify the risk factors associated with PPF in the same cohort. Age or sex could be a risk factor, and the date of polypectomy was matched between the case group and the control group.
The study protocol was approved by the Institutional Review Board of the Asan Medical Center (IRB no. 2013-0172).
After written informed consent was acquired from the patients, all patients were prepared for colonoscopy by asking them to swallow a polyethylene glycol solution before the procedure. All colonoscopic polypectomies were performed by six attending physicians in the Department of Gastroenterology, using standard colonoscopes (CF 240L, or 260L; Olympus Optical Co., Ltd., Tokyo, Japan). Patients were placed under conscious sedation with intravenously administered meperidine and/or midazolam or propofol. Monitoring during colonoscopy and polypectomy included pulse oximetry. Resected material was retrieved using a basket or through simple aspiration into a connection cap, and placed in a numbered container. Each removed polyp was sent to a pathologist for histopathologic examination.
Patients were observed for at least 12 hours after the procedure to detect the occurrence of PPF. If there were no polypectomy-related adverse events such as hemorrhage, perforation, or fever, the patient was discharged the day after the procedure. Each patient was scheduled for a follow-up visit to the outpatient clinic within 2 weeks of the procedure. Those with adverse events underwent additional evaluation and therapeutic procedures.
Patient- and polyp-related factors were investigated to identify the risk factors associated with PPF. Patient-related risk factors included age, sex, comorbid disease (e.g., hypertension, diabetes mellitus, arrhythmia, history of stroke, liver cirrhosis, or a history of abdominal surgery), and medications (e.g., aspirin, clopidogrel, or warfarin). The date of discontinuation of each drug before colonoscopic polypectomy was carefully documented.
Polyp-related variables included the number, size, shape, and histopathology of the polyps. For patients with two or more removed polyps, the size of the largest polyp was recorded. Polyp location was defined as either the left colon (from the rectum to the splenic flexure) or the right colon (from the transverse colon to the cecum). Other polyp-associated variables included the total procedure time for colonoscopy, the method of polyp removal (snare vs. endoscopic mucosal resection [snare after submucosal injection]), experience of colonoscopists performing the polypectomy, and the state of bowel preparation, with the latter also evaluated as a potential risk factor for PPF.7 Bowel preparation states were graded according to the modified Aronchick bowel preparation scale as excellent, good, fair-inadequate, inadequate, or poor.8 Laboratory data, including white blood cell count and C-reactive protein (CRP) levels, were collected for patients with PPF.
Continuous variables were expressed as the median (interquartile range [IQR]) and categorical variables as the number (percentage). Because the number of patients with PPF was small, the Mann-Whitney U and Fisher exact tests were used to analyze continuous and categorical variables, respectively. Binary logistic regression was used to assess the risk factors for PPF. Univariate and multivariable analyses were performed to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) according to select patient- and polyp-related variables. On the basis of the univariate analysis, independent variables with p<0.05 were selected for assessment through multivariable analysis.
Of the 3,444 patients who underwent colonoscopic polypectomy during index hospitalization, 17 (0.49%) developed fever. Ten of these patients were excluded, including five because of postpolypectomy bleeding; two because of microperforation; and one each because of pneumonia, diverticulitis, and Clostridium difficile-associated diarrhea. Thus, of the 3,444 patients, seven (0.2%) met the inclusion criteria. The median peak body temperature was 38.1℃ (range, 37.8 to 38.9). The median interval from colonoscopic polypectomy to the occurrence of fever was 7 hours (IQR 25, 5 hours; IQR 75, 20 hours), and the median duration of fever was 9 hours (IQR 25, 8 hours; IQR 75, 12 hours). Physical examination showed that none of these seven patients had rebound tenderness, with six patients having no tenderness. Blood was drawn for culture from three patients with PPF, but all were negative. Complete blood count and CRP levels were measured in four of the seven patients with PPF. None had elevated CRP levels and three had leukocytosis. Chest radiographs showed that none of the seven patients had new pulmonary parenchymal lesions or intra-abdominal free air.
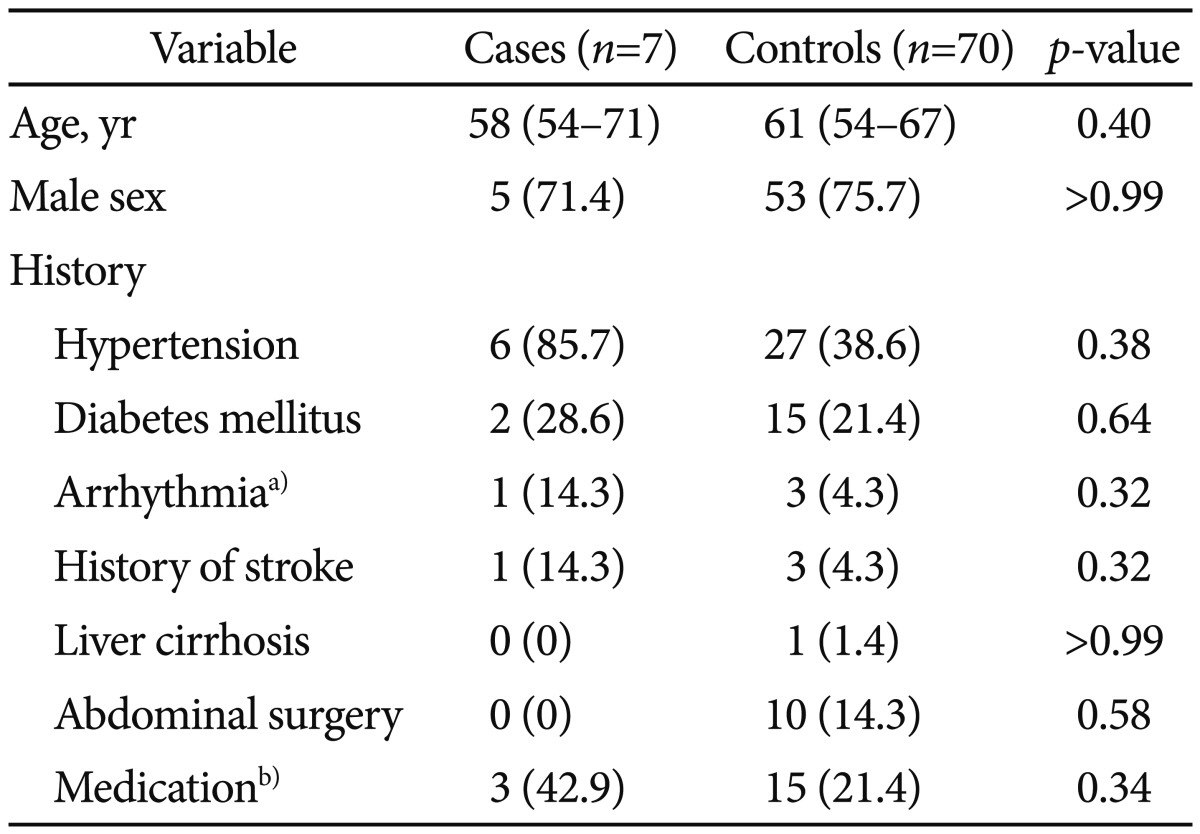
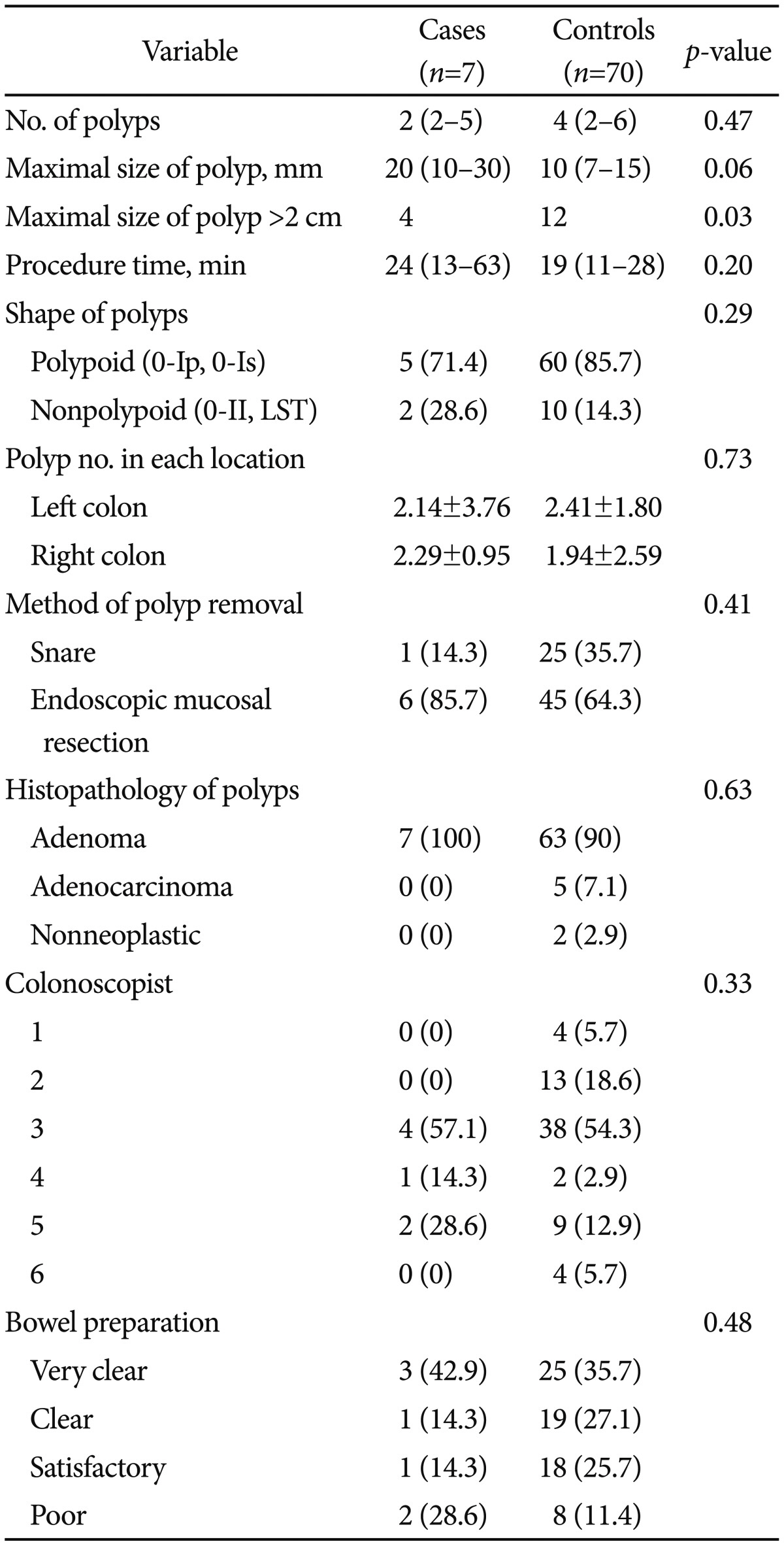
Table 1 shows the baseline characteristics of the patients at enrollment. There were no differences between the case and control groups in terms of age, sex, and medical history. Table 2 shows the polyp-related variables. Maximal polyp size tended to be larger in patients with PPF than in those without PPF (20 mm vs. 10 mm; p=0.065); however, the mean number of polyps in each colon segment was similar in the two groups. There was no significant difference between groups in terms of the median total procedure time (p=0.205) and bowel preparation (p=0.487).
Of the seven patients with PPF, six were treated with antibiotics. Fever subsided within 1 day in all seven cases. The median length of hospitalization was longer for patients with PPF than for those without PPF (3 days vs. 2 days; p=0.03).
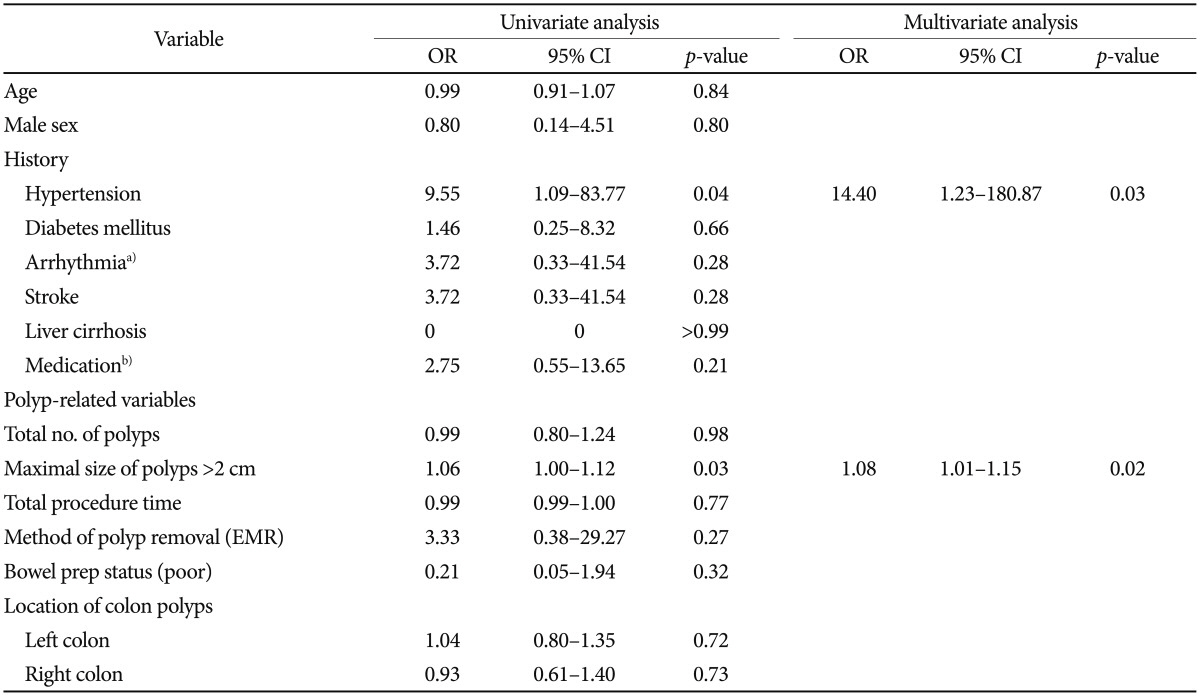
A binary logistic regression model was used to assess the risk factors related to the development of PPF assessed through univariate and multivariate analyses (Table 3). Hypertension (adjusted OR, 14.40; 95% CI, 1.23 to 180.87; p=0.03) and polyp size >2 cm (adjusted OR, 1.08; 95% CI, 1.01 to 1.15; p=0.02) were independent risk factors for PPF.
To our knowledge, this is the first study to assess the incidence, risk factors, and outcomes in patients who developed PPF during index hospitalization. Hypertension and polyps >2 cm were associated with an increased risk of PPF. The length of hospital stay was longer for patients with PPF than for those without PPF.
Fever after colonoscopic polypectomy occurred in 17 of 3,444 (0.49%) inpatients. Although we did not intend to investigate the possible causes of fever after polypectomy, fever was associated with postpolypectomy bleeding in five patients, with microperforation in two patients, and infections associated with other conditions in three patients (one each with pneumonia, diverticulitis, or C. difficile-associated diarrhea). After excluding other possible causes of fever after polypectomy, the crude incidence of PPF was 0.2%. It is unclear whether fever developed because of polypectomy itself or because of colonoscopy. Although the incidence of transient bacteremia after colonoscopy reaches 2.2%,9 signs or symptoms of infection are rare2 and the organisms isolated were skin contaminants in most cases.9 Thus, the incidence of PPF might be lower than that of bacteremia after colonoscopy. However, underestimation of PPF contributed to the lower incidence of PPF because we excluded patients who underwent polypectomy without admission.
Three of the four patients tested showed leukocytosis, but none showed elevated CRP levels. After the initial tissue injury, CRP levels increased up to several hundred-fold within 24 to 48 hours, peaking at 72 hours.10 The median interval from polypectomy to the occurrence of fever in our patients was 7 hours, with blood sampled immediately after the occurrence of PPF. Thus, the CRP concentration was measured <24 hours after the onset of fever, which was too soon to observe any increase.
The median length of hospitalization was significantly longer for patients with PPF than for those without PPF (3 days vs. 2 days; p=0.03). However, there were no PPF-related serious adverse events such as bacteremia, sepsis, local infection, or metastatic infection. Fever subsided within half a day, with a favorable course in all patients with PPF.
Interestingly, the two risk factors for PPF identified in this study, polyp size and hypertension, are the same as those identified in patients with postpolypectomy coagulation syndrome.6 It is unclear whether PPF is the same disease entity as postpolypectomy syndrome, or whether the latter is due to some other mechanism, as discussed above.
Table 1 shows the results of the nonparametric test of demographic and clinical characteristics in cases and controls. However, for Table 3 data, binary logistic regression was performed to assess risk factors and univariate and multivariate analyses were conducted to calculate the ORs. As a result, hypertension was insignificant between the groups in Table 1 (p=0.38); however, in Table 3, hypertension was a significant variable and entered into multivariate analysis.
Our findings do not fully explain the possible mechanisms underlying PPF. However, PPF may be explained by three hypotheses. The first is that PPF is a manifestation of postpolypectomy syndrome, which occurs in 0.07% to 1% of patients who undergo polypectomy, and is thought to result from a transmural burn to the colon wall in the absence of actual perforation.4,6,11 Its presentation includes pain, fever, and localized tenderness, on examination. However, only one of the seven patients in the present study had localized tenderness, and none showed signs of peritoneal irritation. Thus, these patients may have had a milder form of postpolypectomy syndrome.
The second hypothesis is that, during colonoscopic procedures, bacteria translocate from the gut to the bloodstream through a mucosal break. The incidence of transient bacteremia is approximately 3.6% within 10 minutes after polypectomy.12 Bacteremia associated with endoscopic procedures peaks within 5 minutes and then diminishes rapidly within the next 30 to 240 minutes.13 In the present study, however, blood for culturing was obtained immediately after the occurrence of PPF in three of the seven patients, and all were negative. These results are consistent with those of another study that evaluated the frequency of bacteremia associated with an endoscopic mucosal resection or endoscopic submucosal dissection of colon lesions.14 None of these patients was positive for bacteria, and none had signs or symptoms of infection.14
The third hypothesis is that PPF may be caused by an inflammatory mechanism other than infection. Adenomatous polyps have an inflammatory stromal microenvironment, which is rich in macrophages, neutrophils, and T helper cells.15 Moreover, proinflammatory gene expression is high in these polyps, and potent chemoattractants such as interleukin (IL)-8 are relatively abundant.15 Increased polyp size is associated with higher concentrations of proinflammatory cytokines and a greater number of immune cells in the stromal environment, suggesting an association between large polyps and PPF. Histologic examination of the polyps removed from the seven patients in this study showed that six were adenomas and the seventh was an adenocarcinoma.
The mechanism by which hypertension promotes PPF is unclear. Patients with hypertension tend to have innate lowgrade inflammation.16 Also, plasma concentrations of proinflammatory cytokines such as IL-6 and tumor necrosis factor-α are increased in certain subsets of hypertensive patients.17 Thus, patients with hypertension may tend to experience fever caused by local inflammation.
Although PPF could be a mild form of postpolypectomy syndrome based on similar risk factors or similar mechanisms, other plausible mechanisms of sole fever after polypectomy should be considered, such as bacterial translocation and the proinflammatory microenvironment of adenomatous polyps. Thus, PPF would be more appropriate until the precise mechanism is made.
The strength of this study is that it is the first to investigate the incidence of documented PPF in a tertiary hospital. Although some laboratory tests were not performed in all patients, all the medical records were accurately and clearly maintained in the electronic medical records of our center; therefore, accurate chart reviews were available. The results suggest a favorable course of sole fever after colonoscopic polypectomy, and clinicians may need observation time without antibiotic treatment or excessive evaluation.
The limitations of this study include the small number of patients assessed. The number of patients with PPF was too small to identify the risk factors for this condition, as PPF itself is rare. Moreover, the distribution of patient characteristics varied widely. However, an increase in the number of patients may result in a decrease in the OR. Second, the crude incidence of patients with PPF may have been underestimated, as we only enrolled inpatients who developed fever during hospitalization after polypectomy. Thus, we may have missed those patients who experienced fever after discharge. Moreover, we did not include patients who underwent colonoscopic polypectomy in an outpatient clinic and therefore could not be monitored after the procedure. Finally, this study was performed at a single center and thus is not representative of the entire Korean population. However, six experienced attending physicians in our hospital performed these procedures, which might compensate for the limitations of a single-center study.
In previous studies, the rate of colonoscopy-associated bacteremia ranged from 0% to 25%, with a mean frequency of 4.4%.14 Furthermore, those studies were conducted with hospitalized patients, as in our study. There must be several cases of postcolonoscopy fever without polypectomy in nonhospitalized patients. It wound be useful to investigate the incidence and course of postcolonoscopy fever in those nonhospitalized patients, and check if consistent outcomes are obtained, in order to validate our results.
In conclusion, hypertension and polyps >2 cm in size were risk factors for the development of PPF. Although use of prophylactic antibiotics is not warranted, owing to the favorable course of PPF, a greater understanding of the occurrence of PPF and its risk factors is needed for better patient management. To elucidate the mechanism of PPF development, it is necessary to measure the serum concentrations of several cytokines associated with the onset of fever.
References
1. Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993; 329:1977–1981. PMID: 8247072.

2. ASGE Standards of Practice Committee. Fisher DA, Maple JT, et al. Complications of colonoscopy. Gastrointest Endosc. 2011; 74:745–752. PMID: 21951473.

3. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007; 116:1736–1754. PMID: 17446442.
4. Waye JD, Lewis BS, Yessayan S. Colonoscopy: a prospective report of complications. J Clin Gastroenterol. 1992; 15:347–351. PMID: 1294644.
5. Waye JD, Kahn O, Auerbach ME. Complications of colonoscopy and flexible sigmoidoscopy. Gastrointest Endosc Clin N Am. 1996; 6:343–377. PMID: 8673332.

6. Cha JM, Lim KS, Lee SH, et al. Clinical outcomes and risk factors of post-polypectomy coagulation syndrome: a multicenter, retrospective, case-control study. Endoscopy. 2013; 45:202–207. PMID: 23381948.

7. Chilton AP, O'Sullivan M, Cox MA, Loft DE, Nwokolo CU. A blinded, randomized comparison of a novel, low-dose, triple regimen with fleet phospho-soda: a study of colon cleanliness, speed and success of colonoscopy. Endoscopy. 2000; 32:37–41. PMID: 10691270.
8. Gurudu SR, Ratuapli S, Heigh R, DiBaise J, Leighton J, Crowell M. Quality of bowel cleansing for afternoon colonoscopy is influenced by time of administration. Am J Gastroenterol. 2010; 105:2318–2322. PMID: 21048676.

9. Botoman VA, Surawicz CM. Bacteremia with gastrointestinal endoscopic procedures. Gastrointest Endosc. 1986; 32:342–346. PMID: 3533703.

10. Welsch T, Müller SA, Ulrich A, et al. C-reactive protein as early predictor for infectious postoperative complications in rectal surgery. Int J Colorectal Dis. 2007; 22:1499–1507. PMID: 17639424.

11. Fatima H, Rex DK. Minimizing endoscopic complications: colonoscopic polypectomy. Gastrointest Endosc Clin N Am. 2007; 17:145–156. PMID: 17397781.

12. Levy MJ, Norton ID, Clain JE, et al. Prospective study of bacteremia and complications with EUS FNA of rectal and perirectal lesions. Clin Gastroenterol Hepatol. 2007; 5:684–689. PMID: 17544995.

13. Janssen J, König K, Knop-Hammad V, Johanns W, Greiner L. Frequency of bacteremia after linear EUS of the upper GI tract with and without FNA. Gastrointest Endosc. 2004; 59:339–344. PMID: 14997128.

14. Min BH, Chang DK, Kim DU, et al. Low frequency of bacteremia after an endoscopic resection for large colorectal tumors in spite of extensive submucosal exposure. Gastrointest Endosc. 2008; 68:105–110. PMID: 18402955.

15. McLean MH, Murray GI, Stewart KN, et al. The inflammatory microenvironment in colorectal neoplasia. PLoS One. 2011; 6:e15366. PMID: 21249124.

16. Schiffrin EL. The immune system: role in hypertension. Can J Cardiol. 2013; 29:543–548. PMID: 22902155.

17. Granger JP. An emerging role for inflammatory cytokines in hypertension. Am J Physiol Heart Circ Physiol. 2006; 290:H923–H924. PMID: 16467462.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download