Abstract
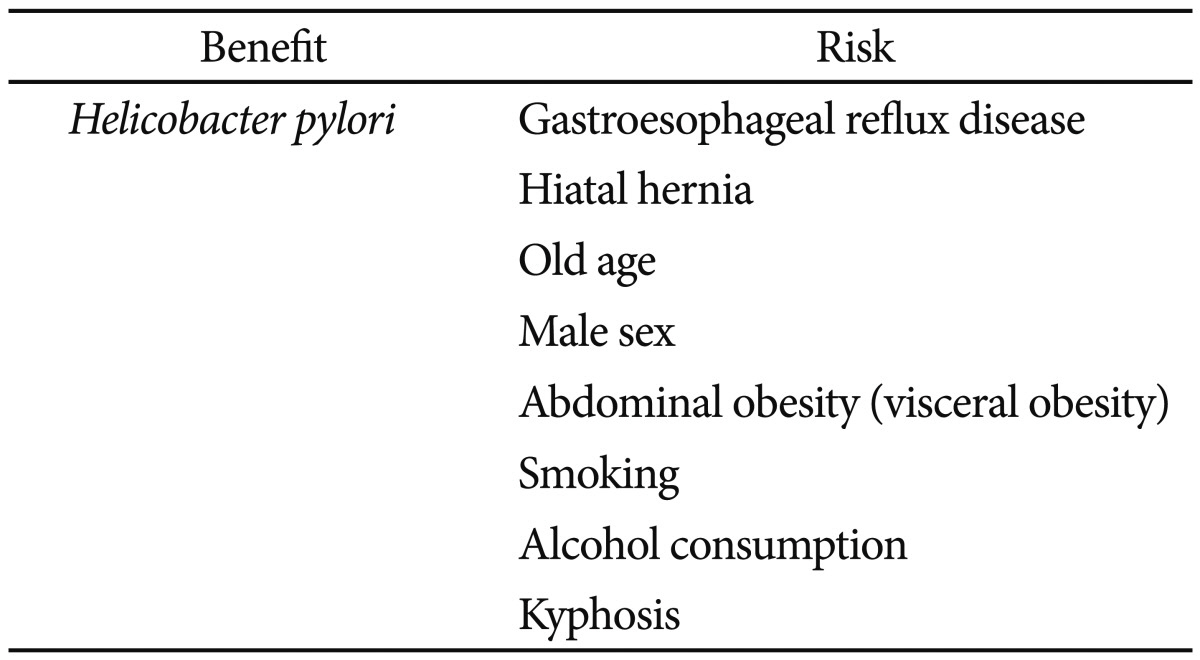
Barrett esophagus (BE) is considered to develop as a result of chronic gastroesophageal reflux disease (GERD) and to predispose to esophageal adenocarcinoma (EAC). However, the disease pattern of BE in Asia differs from that observed in the West. For example, in the West, the prevalence rates of BE and EAC have progressively increased, whereas although the prevalence rate of GERD is increasing in Asia, the prevalence rates of BE and EAC have remained low in most Asian countries. GERD, hiatal hernia, old age, male sex, abdominal obesity (visceral obesity), smoking, alcohol consumption, and kyphosis are known risk factors for BE in Asia, and most Asian patients have short-segment BE. Helicobacter pylori infection is more prevalent in Asia than in the West. We suggest larger studies with a prospective design be conducted to elaborate further the different patterns of BE in Asia.
Barrett esophagus (BE) is defined as a change in the distal esophageal epithelium of any length characterized by columnar type mucosa during endoscopy and is confirmed to have intestinal metaplasia by biopsy.1,2 BE is considered a complication of chronic gastroesophageal reflux disease (GERD)3 and has the potential to develop into esophageal adenocarcinoma (EAC).4 Furthermore, BE is more common in the West than in Asia.5
Although the incidence of GERD has increased in recent years in Asia, BE and EAC are rare.6-9 Based on inferences drawn from epidemiologic data generated in western countries, it is foreseeable that the incidence of BE in Asia could rise.10 Although improvements in endoscopic technology, heightened awareness and interest in BE, and large-scale screening endoscopy programs may increase the reported prevalence of BE, it appears that disease patterns differ between Asia and the West according to published data.
In this report, we discuss the prevalence and risk factors of BE in Asia.
In Western countries, the incidences of BE and EAC have progressively increased since the 1970s.11,12 Between 1994 and 2006, the crude incidence of BE in the United States increased from 14.5 per 100,000 member-years in 1998 to 23.8 per 100,000 member years in 2006 (p-value for trend, <0.01).11 The number of individuals with BE in the United States is difficult to estimate because a substantial proportion of patients are asymptomatic13 and because no consensus regarding screening guidelines has been reached.14
Clinical studies on the prevalence of BE have produced a wide range of estimates. A Swedish study that attempted to approximate a community sample by recruiting volunteers for endoscopic screening estimated a BE population prevalence at 1.6%.15 A similar sized study performed in the United States on patients undergoing screening colonoscopies found a prevalence of 6.8%.16
A recent study used a computer simulation disease model of EAC to determine estimates for BE prevalence that best aligned with the United States Surveillance Epidemiology and End Results (SEER) cancer registry data. This model consists of six health states: normal, GERD, BE, undetected cancer, detected cancer, and death. Published literature regarding the transition rates between these states was used to provide boundaries. Using this model, the estimated prevalence for BE in the general population was 5.6% (range, 5.49 to 5.70) in the United States, and the model accurately predicted incidence rates (4.5 of 100,000 in 1975 to 2005) for EAC reported to the United States SEER cancer registry.17
A study on the incidence and prevalence of BE was conducted on approximately 3.3 million persons in Northern California in 2007. The prevalence of diagnosed BE rose steadily throughout the study period from 1994 to 2006 and reached 131 per 100,000 member-years in 2006 in the adult population. Furthermore, prevalence increased with age (440/100,000 member-years for 81 to 90-year-olds in 2006), and it was much higher among men than among women (179 vs. 87 diagnoses/100,000 sex-specific member-years in 2006). In addition, the prevalence among non-Hispanic whites in 2006 was 2-fold higher than that among Hispanic whites (247 vs. 135/100,000 race-specific member-years, respectively) and approximately 5-fold higher than that among black Americans (49/100,000 member-years) and Asian Americans (65/100,000 member-years).11 Thus, it appears that the prevalence of BE increases with age in the West.
The prevalence of GERD is lower in Asia than in the West. It was estimated that the approximate prevalence of GERD in the West is 10% to 20% and that its rate is substantially lower in Asia at <5%.18 Thus, it might be expected that the complications of GERD, such as BE, are less prevalent in Asia than in the West.19 The reported prevalence of BE in Asia shows that the disease is still rare in most parts of Asia (Table 1).20-34 The prevalence of BE in a Chinese population undergoing upper gastrointestinal endoscopy in Taiwan between 1991 and 1992 was only 2% initially.22
A 7-year descriptive study performed in Eastern China from 2005 to 2012 showed that the prevalence of BE remains low in this region. Only 234 of 139,416 patients (0.168%) undergoing gastroscopy were found to have BE. In this study, the mean subject age was 61 years, and the male-to-female ratio was 1.25:1. The prevalence was found to increase with age, and most BE cases were confined to the lower esophagus (92.7%) and short-segment (SS) type. GERD symptoms were present in 131 patients (56%).23
In another Chinese study, BE was found in 1.0% of 2,022 patients who underwent endoscopy in 2007.21 Other studies conducted in China have also shown that BE remained infrequent with a prevalence of 0.06% in the general population and of <2% in referral patients. Advancing age and hiatal hernia were confirmed independent risk factors. Most cases were SS BE, and long-segment BE was uncommon, especially in women. The incidence of EAC was very low in China; it accounted for only approximately 1% of all distal esophageal cancers.9,21,23,24
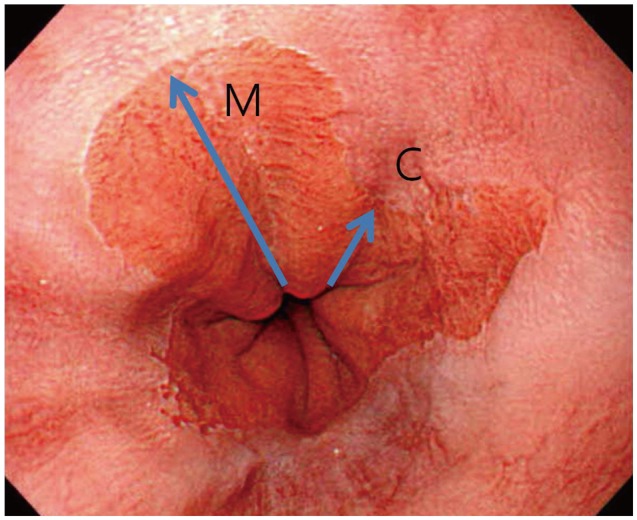
On the other hand, the prevalence of BE was reported to be as high as 19.9% in Japan in a series where biopsy was employed25 and as high as 43.0% in series without biopsy (Fig. 1).26,27 However, the different definitions used for the esophagogastric junction (EGJ) might, to a large extent, account for this discrepancy. Most studies conducted in Asia outside of Japan defined the proximal margin of gastric folds as the EGJ, whereas the Japanese studies used the distal margin of the palisade vessels as the EGJ landmark.35
In Korea, Choi et al.20 found a 1.0% prevalence of BE among 4,002 patients investigated by screening endoscopy from 2010 to 2012. Furthermore, a Korean nationwide prospective multicenter study showed that the prevalence of BE remains low in the Korean population. BE was diagnosed in 1% of 2,048 patients, and a multivariate analysis showed that the risk factors for BE were the presence of typical reflux symptoms and reflux esophagitis.28 In Asian patients, esophagitis exists, but BE is infrequently diagnosed, and most BE cases are the SS type.9,27,29
In Asia, prevalence data, particularly those derived solely from endoscopic diagnosis, vary considerably. Furthermore, endoscopic diagnostic criteria (Table 2)8,21,26,28,29,31,36,37,38 and pathologic diagnostic criteria (Table 3)25,33,39-41 differed markedly from region to region.42 In addition, the biopsy protocols have not been standardized, and the endoscopic diagnosis of SS BE, particularly of segment length <1 cm, is difficult and highly unreliable.43
Evidence regarding the reliability of the endoscopic diagnosis of BE is extremely limited in Asia.10 The Asia Barrett's Consortium recently conducted a multinational trial using video clips of patients with BE to determine interobserver reliability for BE grading by Asian endoscopists.44 The study was performed following rigorous training of endoscopists with respect to the use of the Prague C&M classification (Fig. 2) for BE diagnosis and grading.45 Reliability coefficients for the recognition of BE extending ≥1 cm were 0.90 (range, 0.80 to 1.00) and 0.92 (range, 0.87 to 0.98) for Prague C&M values, respectively, but were markedly lower (0.18 [range, 0.03 to 0.32] and 0.21 [range, 0.00 to 0.51], respectively) for BE segments <1 cm. Accordingly, it was concluded that the endoscopic diagnosis of BE has an unacceptably low interobserver reliability for very short segment disease (<1 cm).44 In a similar study conducted in the West, we identified an abrupt drop in reliability coefficients when assessing videos with mean C&M values <1 cm. The consistency of this finding in Asia and the West suggests that SS BE is difficult to diagnose consistently.45-47
Studies conducted to date have several limitations. For example, study populations were not representative of general populations, as most studies were performed in single tertiary care referral centers. Furthermore, the majority of Asian studies did not use a standardized 4-quadrant biopsy protocol, and this is likely to have resulted in an underestimation of the prevalence of BE. To overcome these limitations and to more accurately estimate and compare the prevalence of BE in Asia, consensus should be reached on the biopsy protocol, endoscopic diagnostic criteria, and pathologic diagnostic criteria.10
BE is clinically important because of the risk of progression to adenocarcinoma. The incidence of EAC has increased significantly in the West.48,49 A meta-analysis based on 47 studies showed that the pooled estimate for cancer incidence in BE was 6.1 per 1,000 person-years. Men progressed to cancer at twice the rate of women.50
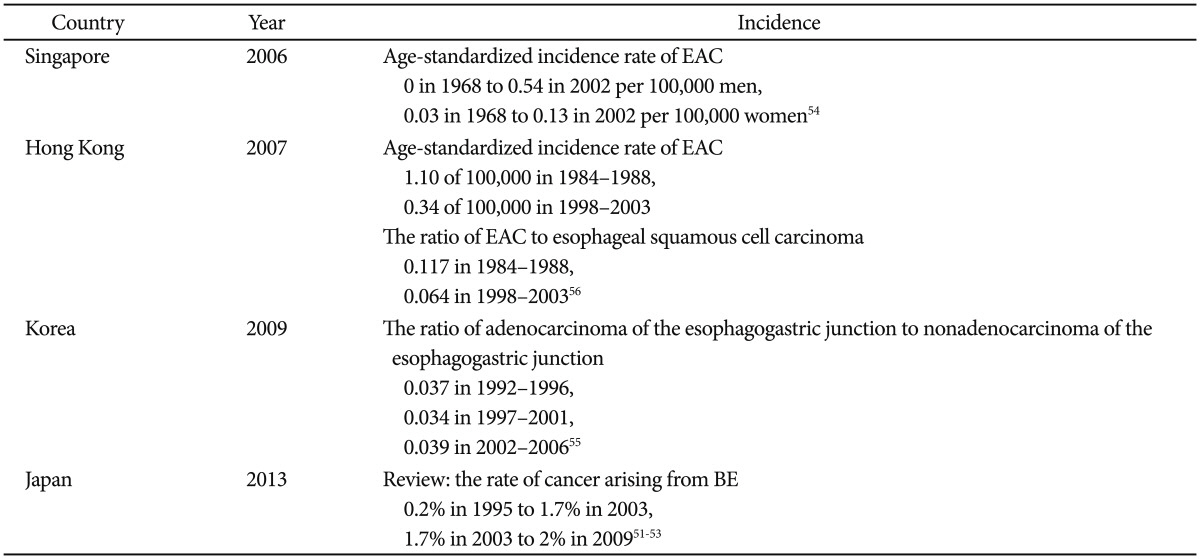
Unlike the increasing trend of EAC in the West, the disease trend is less clear in Asia. The incidence of EAC is increasing slowly in Japan51-53 and in Singapore,54 remains unchanged in Korea,55 and is declining in Hong Kong (Table 4).56
In Japan, according to national statistics on esophageal cancer published by the Japanese Esophageal Society, the rate of cancer arising from BE increased from 0.2% in 1995 to 1.7% in 2003.51 The data of the Japanese Association for Thoracic Surgery indicated a smaller change in the rate of cancer arising from BE, from approximately 1.7% in 2003 to 2% in 2009.51-53 Although the incidence of EAC appears to have increased from 1995 to 2009, the increase has not been as dramatic as the increases observed in Europe and the United States.51
There appears to be a trend toward an increase in the incidence of EAC in Singapore, although the absolute incidence remains relatively low. Data obtained from the Singapore Cancer Registry and population data derived from national censuses showed that the age-standardized incidence rates for EAC rose from 0 to 0.54 per 100,000 men and from 0.03 to 0.13 per 100,000 women between 1968 and 2002. This may be caused by an associated increase in the frequency of reflux esophagitis and obesity in Singapore.54
In Korea, a retrospective review of the medical records of 16,811 patients diagnosed with esophageal cancer or gastric adenocarcinoma between 1992 and 2006 showed no increase in the incidence ratio of adenocarcinoma of the EGJ to nonadenocarcinoma of the EGJ.55
In Hong Kong, a study was conducted using population-based data of the Hong Kong Cancer Registry from 1984 to 2003. This study of 10,751 new cases of esophageal neoplasm showed that EAC declined among men and women; total numbers decreased from 224 in 1984 to 1988 to 131 in 1998 to 2003. Furthermore, these declines were faster than those of esophageal squamous cell carcinoma; thus, the ratio of EAC to esophageal squamous cell carcinoma decreased from 11.7% in 1984 to 1988 to 6.4% in 1998 to 2003 despite increasing prevalence of GERD and other risk factors.56
Although the incidence of EAC has increased in Asia, these increases have been only slight. This might be explained by the fact that most BE cases in Asia are of the SS type and that this condition is associated with a limited risk of EAC. Regardless of whether or not the incidence of EAC is increasing, monitoring and surveillance data are needed on the general population in Asia.
Surveillance is controversial because of the lack of randomized trials supporting its value. There are concerns about the cost-effectiveness given the low incidence rates of EAC. Cost-effectiveness studies suggest that BE surveillance was at least as effective as other widely accepted medical practices.19 The cost-effectiveness of endoscopic surveillance of BE was compared with screening mammography in a cohort study. The incidence of EAC was one case per 73 patient-years of follow-up, whereas occult breast cancer was detected in 50 of 12,537 mammograms. The costs of detecting a case of EAC and occult breast cancer were $37,928 and $54,513, respectively, and costs for treatment resulting in cure were $83,340 and $83,292, respectively. Cost per life-year saved was $4,151 for EAC and $57,926 for breast cancer. Thus, endoscopic surveillance of patients with BE was as cost-effective as surveillance mammography.57 Another study used newer estimates of cancer risk, which was lower at 0.4% cases per year. The model evaluated surveillance every 1 to 5 years and no surveillance, with esophagectomy performed if high-grade intraepithelial neoplasia was diagnosed, and calculated the incremental cost-utility ratios for each strategy. The results suggested that 5-yearly surveillance was the only viable strategy. More frequent surveillance was more expensive and yielded a lower life expectancy. The incremental cost-utility ratio for 5-yearly surveillance was $98,000 per quality-adjusted life-year gained, comparable with other accepted practices like heart transplantation ($160,000) and tuberculosis screening ($216,000).58 However, it remains to be determined how best to improve the cost-effectiveness of surveillance strategies in patients with BE.19
BE is clearly associated with severe GERD. Compared with patients with erosive or nonerosive GERD without BE, patients with BE typically have greater esophageal acid exposure, as determined by 24-hour pH monitoring.59-61
The reason for the increase in acid exposure in Barrett's patients may be related to the almost uniform presence of hiatal hernia, which is typically longer and associated with larger defects in the hiatus than in controls or in patients with esophagitis alone.62 There is a strong association between BE and the presence of hiatal hernia in Asia and in the West.38,62,63 In addition, patients with BE have lower basal esophageal sphincter pressure than GERD patients without BE.61
BE is not related to gastric acid hypersecretion because studies performed using appropriate controls found no differences between basal acid outputs, gastrin-stimulated peak acid outputs, overnight fasting residues, and pepsin outputs in BE.64 However, reflux of duodenal contents is greater in BE patients than in age-matched controls and GERD patients without BE.65,66
Helicobacter pylori is more prevalent in Asia than in the West, and most Asian studies conducted on the subject have shown that this bacterium has a protective effect on BE.66 A systematic review of 20 studies found that the prevalence of H. pylori infection was significantly lower in patients with GERD than in those without GERD and that geographical location strongly contributed to heterogeneity between studies. Patients with GERD in the Far East were also reported to have a lower prevalence of H. pylori infection than those in Western Europe or North America, despite a higher prevalence in the general population,67 and in a Japanese study, most BE patients were found to be free of H. pylori infection.68
Old age and male sex are significant risk factors for BE in Asia. The mean age of BE patients was 53.8 years in a retrospective analysis of 70,103 Korean patients,29 and in a Japanese study, mean ages for circumferential BE and tongue-like BE were 56.6 and 62.5 years, respectively.27 Although the overall prevalence rate of BE is lower in the East than in the West, the prevalence rates of longer duration GERD symptoms, old age, and male sex are similar among patients with BE in the East and West.9,39,40,69
In a systemic literature review of 10 case-control studies conducted in the West, four studies concluded that an increased body mass index (BMI) was associated with a greater risk of BE,70 whereas a retrospective cohort study in the Japanese general population showed that simple obesity was not a risk factor of BE.26
In another retrospective cohort study in Japanese patients with nonalcoholic fatty liver disease, the effect of simple obesity, as measured by BMI, and visceral obesity (defined by the surface area of visceral adipose tissue as calculated from abdominal computed tomography images) on the risk of BE was analyzed.71 The association between BMI and BE did not reach statistical significance, whereas the area of visceral adipose tissue was independently associated with the risk of BE, after adjusting for BMI. These findings suggest that the effect of obesity on the risk of BE is mainly mediated by abdominal obesity, especially in the visceral fat area, rather than by simple obesity.72
Differences between the strengths of the association between BMI and the risk of BE in Western and Japanese reports may be explained, at least in part, by ethnic obesity pattern differences, especially by different patterns of visceral adipose tissue deposition. Abdominal obesity can explain, to some extent, the epidemiological features of BE and EAC. For example, body fat distribution tends more toward visceral obesity than simple obesity in groups at high risk of BE, such as Caucasians as compared with Asians, and men as compared with women.72,73
Two Asian studies showed that smoking is a significant risk factor of BE.26,29 In a Japanese study of 463 men, it was suggested that alcohol consumption is associated with an increased risk of erosive esophagitis and BE.74 In a Korean study on the risk factors associated with erosive esophagitis, smoking and alcohol drinking were found to be significantly associated by univariate analysis, and alcohol drinking remained a risk factor after adjustment by multivariate logistic analysis.75 On the other hand, smoking and alcohol consumption were not found to be associated with the development of BE in Western patients.76 A retrospective study in Japanese patients showed that kyphosis is a risk factor for the presence of long-segment BE.77 Significant differences in the Cobb angle, a marker of kyphosis, were found between long-segment BE and control patients (with SS BE or without BE).
In summary, H. pylori infection shows an inverse association with BE. GERD, hiatal hernia, old age, male sex, abdominal obesity (visceral obesity), smoking, and alcohol consumption are risk factors of BE (Table 5).
Identification of factors that predict the elongation of Barrett's mucosa may provide valuable insight into treatment and endoscopic surveillance.78 SS BE without pathological confirmation is called SS columnar-lined esophagus (CLE) and is diagnosed in patients with columnar epithelium covering <3 cm of at least one segment of the distal esophagus. Manabe et al.78 investigated the chronological changes of SS-CLE. SS-CLE showed elongation in approximately 5.8% of patients after a mean follow-up period of 5.7 years. SS-CLE remains stable in length over time. In patients with SS-CLE, the predictors of its elongation were the absence of endoscopic atrophic gastritis, the presence of reflux esophagitis, and the presence of flame-shaped SS-CLE at the initial examination.78
In China, Huang et al.79 proposed a clinical practice recommendation to optimize appropriate management to patients, promote clinical research among different centers, and prevent EAC. Antacid therapy should be used for symptomatic BE patients without dysplasia, and endoscopic surveillance with biopsies every 2 to 3 years is recommended for those with several risk factors such as male sex, age >40 years, hiatal hernia, severe GERD >5 years, and morbid obesity. If the biopsies for two consecutive surveillance endoscopies are negative, the endoscopic surveillance interval is extended to 3 to 5 years. For low-grade dysplasia, endoscopic surveillance with conventional white light endoscopy is recommended to perform a 4-quadrant biopsy with every 1 cm in CLE. For high-grade dysplasia and intramucosal carcinoma, an endoscopic or surgical intervention is suggested.79
To determine the best treatment for superficial BE-associated EAC, 12 expert endoscopists and a pathologist from the Asia Pacific region conducted a session in Japan.80 After a discussion, they proposed consensus statements on endoscopic diagnosis and treatment of superficial EAC as follows. Representative characteristics by conventional white light endoscopy are a reddish area or a lesion located on the anterior to right side wall. Image-enhanced endoscopy may not be very helpful in locating the EAC but could be useful for characterizing the tumor and diagnosing lateral tumor extension, especially when using narrow band imaging or an acetic acid-spraying method. Endoscopic mucosal resection or endoscopic submucosal dissection for mucosal carcinomas could provide excellent prognosis.80
The prevalence of GERD and related esophagitis in some parts of Asia has increased markedly recently. Based on epidemiologic data generated in Western countries, it is foreseeable that the incidence of BE and EAC in Asia could rise.10
However, as previously described, the evolving disease pattern of BE in Asia may differ from that experienced in the West. Furthermore, studies conducted in Asia are limited by small study populations and differences between the biopsy protocols, endoscopic diagnostic criteria, and pathologic diagnostic criteria used to estimate and compare the prevalence and risk factors of BE in Asia. Accordingly, we suggest larger studies with a prospective design be conducted to examine this issue and to elaborate further the different patterns of BE in Asia.
References
1. Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett's esophagus: the AGA Chicago Workshop. Gastroenterology. 2004; 127:310–330. PMID: 15236196.
2. Wang KK, Sampliner RE. Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2008; 103:788–797. PMID: 18341497.

3. Lieberman DA, Oehlke M, Helfand M. Gastroenterology Outcomes Research Group in Endoscopy. Risk factors for Barrett's esophagus in community-based practice. GORGE consortium. Am J Gastroenterol. 1997; 92:1293–1297. PMID: 9260792.
4. Hameeteman W, Tytgat GN, Houthoff HJ, van den Tweel JG. Barrett's esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989; 96(5 Pt 1):1249–1256. PMID: 2703113.

6. Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009; 24:729–735. PMID: 19646015.

7. Ho KY, Cheung TK, Wong BC. Gastroesophageal reflux disease in Asian countries: disorder of nature or nurture? J Gastroenterol Hepatol. 2006; 21:1362–1365. PMID: 16911677.

8. Azuma N, Endo T, Arimura Y, et al. Prevalence of Barrett's esophagus and expression of mucin antigens detected by a panel of monoclonal antibodies in Barrett's esophagus and esophageal adenocarcinoma in Japan. J Gastroenterol. 2000; 35:583–592. PMID: 10955596.

9. Chen X, Zhu LR, Hou XH. The characteristics of Barrett's esophagus: an analysis of 4120 cases in China. Dis Esophagus. 2009; 22:348–353. PMID: 19191861.

10. Chang CY, Cook MB, Lee YC, et al. Current status of Barrett's esophagus research in Asia. J Gastroenterol Hepatol. 2011; 26:240–246. PMID: 21155883.
11. Corley DA, Kubo A, Levin TR, et al. Race, ethnicity, sex and temporal differences in Barrett's oesophagus diagnosis: a large community-based study, 1994-2006. Gut. 2009; 58:182–188. PMID: 18978173.

12. Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991; 265:1287–1289. PMID: 1995976.

13. Chak A, Faulx A, Eng C, et al. Gastroesophageal reflux symptoms in patients with adenocarcinoma of the esophagus or cardia. Cancer. 2006; 107:2160–2166. PMID: 17019737.

14. Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. JAMA. 2002; 287:1972–1981. PMID: 11960540.
15. Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology. 2005; 129:1825–1831. PMID: 16344051.

16. Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003; 125:1670–1677. PMID: 14724819.

17. Hayeck TJ, Kong CY, Spechler SJ, Gazelle GS, Hur C. The prevalence of Barrett's esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010; 23:451–457. PMID: 20353441.
18. Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005; 54:710–717. PMID: 15831922.

19. Fock KM, Ang TL. Global epidemiology of Barrett's esophagus. Expert Rev Gastroenterol Hepatol. 2011; 5:123–130. PMID: 21309677.

20. Choi CY, Suh S, Park JS, et al. The prevalence of Barrett's esophagus and the comparison of Barrett's esophagus with cardiac intestinal metaplasia in the health screening at a secondary care hospital. Korean J Gastroenterol. 2012; 60:219–223. PMID: 23089907.

21. Xiong LS, Cui Y, Wang JP, et al. Prevalence and risk factors of Barrett's esophagus in patients undergoing endoscopy for upper gastrointestinal symptoms. J Dig Dis. 2010; 11:83–87. PMID: 20402833.

22. Yeh C, Hsu CT, Ho AS, Sampliner RE, Fass R. Erosive esophagitis and Barrett's esophagus in Taiwan: a higher frequency than expected. Dig Dis Sci. 1997; 42:702–706. PMID: 9125635.
23. Zhang M, Fan XS, Zou XP. The prevalence of Barrett's esophagus remains low in Eastern China. Single-center 7-year descriptive study. Saudi Med J. 2012; 33:1324–1329. PMID: 23232681.
24. Peng S, Cui Y, Xiao YL, et al. Prevalence of erosive esophagitis and Barrett's esophagus in the adult Chinese population. Endoscopy. 2009; 41:1011–1017. PMID: 19967617.

25. Amano Y, Kushiyama Y, Yuki T, et al. Prevalence of and risk factors for Barrett's esophagus with intestinal predominant mucin phenotype. Scand J Gastroenterol. 2006; 41:873–879. PMID: 16803684.

26. Akiyama T, Inamori M, Akimoto K, et al. Risk factors for the progression of endoscopic Barrett's epithelium in Japan: a multivariate analysis based on the Prague C & M Criteria. Dig Dis Sci. 2009; 54:1702–1707. PMID: 19003532.
27. Okita K, Amano Y, Takahashi Y, et al. Barrett's esophagus in Japanese patients: its prevalence, form, and elongation. J Gastroenterol. 2008; 43:928–934. PMID: 19107336.

28. Lee IS, Choi SC, Shim KN, et al. Prevalence of Barrett's esophagus remains low in the Korean population: nationwide cross-sectional prospective multicenter study. Dig Dis Sci. 2010; 55:1932–1939. PMID: 19798574.

29. Kim JH, Rhee PL, Lee JH, et al. Prevalence and risk factors of Barrett's esophagus in Korea. J Gastroenterol Hepatol. 2007; 22:908–912. PMID: 17565647.

30. Dhawan PS, Alvares JF, Vora IM, et al. Prevalence of short segments of specialized columnar epithelium in distal esophagus: association with gastroesophageal reflux. Indian J Gastroenterol. 2001; 20:144–147. PMID: 11497172.
31. Rosaida MS, Goh KL. Gastro-oesophageal reflux disease, reflux oesophagitis and non-erosive reflux disease in a multiracial Asian population: a prospective, endoscopy based study. Eur J Gastroenterol Hepatol. 2004; 16:495–501. PMID: 15097043.
32. Odemiş B, Ciçek B, Zengin NI, et al. Barrett's esophagus and endoscopically assessed esophagogastric junction integrity in 1000 consecutive Turkish patients undergoing endoscopy: a prospective study. Dis Esophagus. 2009; 22:649–655. PMID: 19515192.
33. Chang CY, Lee YC, Lee CT, et al. The application of Prague C and M criteria in the diagnosis of Barrett's esophagus in an ethnic Chinese population. Am J Gastroenterol. 2009; 104:13–20. PMID: 19098843.

34. Khamechian T, Alizargar J, Mazoochi T. The prevalence of Barrett's esophagus in outpatients with dyspepsia in Shaheed Beheshti Hospital of Kashan. Iran J Med Sci. 2013; 38:263–266. PMID: 24174698.
35. Amano Y, Ishimura N, Furuta K, et al. Which landmark results in a more consistent diagnosis of Barrett's esophagus, the gastric folds or the palisade vessels? Gastrointest Endosc. 2006; 64:206–211. PMID: 16860070.

36. Choi DW, Oh SN, Baek SJ, et al. Endoscopically observed lower esophageal capillary patterns. Korean J Intern Med. 2002; 17:245–248. PMID: 12647639.

37. Kuo CJ, Lin CH, Liu NJ, Wu RC, Tang JH, Cheng CL. Frequency and risk factors for Barrett's esophagus in Taiwanese patients: a prospective study in a tertiary referral center. Dig Dis Sci. 2010; 55:1337–1343. PMID: 19557516.

38. Fujiwara Y, Higuchi K, Shiba M, et al. Association between gastroesophageal flap valve, reflux esophagitis, Barrett's epithelium, and atrophic gastritis assessed by endoscopy in Japanese patients. J Gastroenterol. 2003; 38:533–539. PMID: 12825128.

39. Park JJ, Kim JW, Kim HJ, et al. The prevalence of and risk factors for Barrett's esophagus in a Korean population: a nationwide multicenter prospective study. J Clin Gastroenterol. 2009; 43:907–914. PMID: 19417682.
40. Tseng PH, Lee YC, Chiu HM, et al. Prevalence and clinical characteristics of Barrett's esophagus in a Chinese general population. J Clin Gastroenterol. 2008; 42:1074–1079. PMID: 18360296.

41. Rajendra S, Kutty K, Karim N. Ethnic differences in the prevalence of endoscopic esophagitis and Barrett's esophagus: the long and short of it all. Dig Dis Sci. 2004; 49:237–242. PMID: 15104363.

42. Takubo K, Vieth M, Aida J, et al. Differences in the definitions used for esophageal and gastric diseases in different countries: endoscopic definition of the esophagogastric junction, the precursor of Barrett's adenocarcinoma, the definition of Barrett's esophagus, and histologic criteria for mucosal adenocarcinoma or high-grade dysplasia. Digestion. 2009; 80:248–257. PMID: 19828957.
43. Ho KY. From GERD to Barrett's esophagus: is the pattern in Asia mirroring that in the West? J Gastroenterol Hepatol. 2011; 26:816–824. PMID: 21265879.

44. Lee YC, Cook MB, Bhatia S, et al. Interobserver reliability in the endoscopic diagnosis and grading of Barrett's esophagus: an Asian multinational study. Endoscopy. 2010; 42:699–704. PMID: 20806154.

45. Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology. 2006; 131:1392–1399. PMID: 17101315.
46. Spechler SJ. Short and ultrashort Barrett's esophagus: what does it mean? Semin Gastrointest Dis. 1997; 8:59–67. PMID: 9109693.
47. Derakhshan MH, Malekzadeh R, Watabe H, et al. Combination of gastric atrophy, reflux symptoms and histological subtype indicates two distinct aetiologies of gastric cardia cancer. Gut. 2008; 57:298–305. PMID: 17965056.

48. Parfitt JR, Miladinovic Z, Driman DK. Increasing incidence of adenocarcinoma of the gastroesophageal junction and distal stomach in Canada: an epidemiological study from 1964-2002. Can J Gastroenterol. 2006; 20:271–276. PMID: 16609756.
49. Lagergren J. Adenocarcinoma of oesophagus: what exactly is the size of the problem and who is at risk? Gut. 2005; 54(Suppl 1):i1–i5. PMID: 15711002.

50. Yousef F, Cardwell C, Cantwell MM, Galway K, Johnston BT, Murray L. The incidence of esophageal cancer and high-grade dysplasia in Barrett's esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008; 168:237–249. PMID: 18550563.

51. Miyazaki T, Inose T, Tanaka N, et al. Management of Barrett's esophageal carcinoma. Surg Today. 2013; 43:353–360. PMID: 23283352.

52. Committee for Scientific Affairs. Sakata R, Fujii Y, Kuwano H. Thoracic and cardiovascular surgery in Japan during 2009: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2011; 59:636–667. PMID: 22231795.

53. Kazui T, Wada H, Fujita H. Japanese Association for Thoracic Surgery Committee of Science. Thoracic and cardiovascular surgery in Japan during 2003: annual report by The Japanese Association for Thoracic Surgery. Jpn J Thorac Cardiovasc Surg. 2005; 53:517–536. PMID: 16200897.
54. Fernandes ML, Seow A, Chan YH, Ho KY. Opposing trends in incidence of esophageal squamous cell carcinoma and adenocarcinoma in a multi-ethnic Asian country. Am J Gastroenterol. 2006; 101:1430–1436. PMID: 16863543.

55. Chung JW, Lee GH, Choi KS, et al. Unchanging trend of esophagogastric junction adenocarcinoma in Korea: experience at a single institution based on Siewert's classification. Dis Esophagus. 2009; 22:676–681. PMID: 19222529.

56. Yee YK, Cheung TK, Chan AO, Yuen MF, Wong BC. Decreasing trend of esophageal adenocarcinoma in Hong Kong. Cancer Epidemiol Biomarkers Prev. 2007; 16:2637–2640. PMID: 18086768.

57. Streitz JM Jr, Ellis FH Jr, Tilden RL, Erickson RV. Endoscopic surveillance of Barrett's esophagus: a cost-effectiveness comparison with mammographic surveillance for breast cancer. Am J Gastroenterol. 1998; 93:911–915. PMID: 9647017.

58. Provenzale D, Schmitt C, Wong JB. Barrett's esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999; 94:2043–2053. PMID: 10445526.

59. Neumann CS, Cooper BT. 24 hour ambulatory oesophageal pH monitoring in uncomplicated Barrett's oesophagus. Gut. 1994; 35:1352–1355. PMID: 7959184.

60. Oberg S, DeMeester TR, Peters JH, et al. The extent of Barrett's esophagus depends on the status of the lower esophageal sphincter and the degree of esophageal acid exposure. J Thorac Cardiovasc Surg. 1999; 117:572–580. PMID: 10047662.
61. Singh P, Taylor RH, Colin-Jones DG. Esophageal motor dysfunction and acid exposure in reflux esophagitis are more severe if Barrett's metaplasia is present. Am J Gastroenterol. 1994; 89:349–356. PMID: 8122643.
62. Cameron AJ. Barrett's esophagus: prevalence and size of hiatal hernia. Am J Gastroenterol. 1999; 94:2054–2059. PMID: 10445527.

63. Rajendra S, Ackroyd R, Robertson IK, Ho JJ, Karim N, Kutty KM. Helicobacter pylori, ethnicity, and the gastroesophageal reflux disease spectrum: a study from the East. Helicobacter. 2007; 12:177–183. PMID: 17309756.

64. Hirschowitz BI. Gastric acid and pepsin secretion in patients with Barrett's esophagus and appropriate controls. Dig Dis Sci. 1996; 41:1384–1391. PMID: 8689915.

65. Champion G, Richter JE, Vaezi MF, Singh S, Alexander R. Duodenogastroesophageal reflux: relationship to pH and importance in Barrett's esophagus. Gastroenterology. 1994; 107:747–754. PMID: 8076761.

66. Vaezi MF, Richter JE. Role of acid and duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology. 1996; 111:1192–1199. PMID: 8898632.

67. Raghunath A, Hungin AP, Wooff D, Childs S. Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: systematic review. BMJ. 2003; 326:737. PMID: 12676842.

68. Abe Y, Iijima K, Koike T, et al. Barrett's esophagus is characterized by the absence of Helicobacter pylori infection and high levels of serum pepsinogen I concentration in Japan. J Gastroenterol Hepatol. 2009; 24:129–134. PMID: 19196398.
69. Hongo M. Review article: Barrett's oesophagus and carcinoma in Japan. Aliment Pharmacol Ther. 2004; 20(Suppl 8):50–54. PMID: 15575874.

70. Seidel D, Muangpaisan W, Hiro H, Mathew A, Lyratzopoulos G. The association between body mass index and Barrett's esophagus: a systematic review. Dis Esophagus. 2009; 22:564–570. PMID: 19392850.

71. Akiyama T, Yoneda M, Inamori M, et al. Visceral obesity and the risk of Barrett's esophagus in Japanese patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2009; 9:56. PMID: 19622165.

72. Akiyama T, Yoneda M, Maeda S, Nakajima A, Koyama S, Inamori M. Visceral obesity and the risk of Barrett's esophagus. Digestion. 2011; 83:142–145. PMID: 21266807.

73. Weinsier RL, Hunter GR, Gower BA, Schutz Y, Darnell BE, Zuckerman PA. Body fat distribution in white and black women: different patterns of intraabdominal and subcutaneous abdominal adipose tissue utilization with weight loss. Am J Clin Nutr. 2001; 74:631–636. PMID: 11684531.

74. Akiyama T, Inamori M, Iida H, et al. Alcohol consumption is associated with an increased risk of erosive esophagitis and Barrett's epithelium in Japanese men. BMC Gastroenterol. 2008; 8:58. PMID: 19077221.

75. Lee SJ, Jung MK, Kim SK, et al. Clinical characteristics of gastroesophageal reflux disease with esophageal injury in korean: focusing on risk factors. Korean J Gastroenterol. 2011; 57:281–287. PMID: 21623136.

76. Caygill CP, Johnston DA, Lopez M, et al. Lifestyle factors and Barrett's esophagus. Am J Gastroenterol. 2002; 97:1328–1331. PMID: 12094845.

77. Uno G, Amano Y, Yuki T, et al. Relationship between kyphosis and Barrett's esophagus in Japanese patients. Intern Med. 2011; 50:2725–2730. PMID: 22082882.

78. Manabe N, Haruma K, Imamura H, et al. Does short-segment columnar-lined esophagus elongate during a mean follow-up period of 5.7 years? Dig Endosc. 2011; 23:166–172. PMID: 21429023.
79. Huang Q, Fang DC, Fang JY, et al. How to diagnose and manage patients with Barrett's esophagus in China. J Dig Dis. 2012; 13:123–132. PMID: 22356307.

80. Goda K, Singh R, Oda I, et al. Current status of endoscopic diagnosis and treatment of superficial Barrett's adenocarcinoma in Asia-Pacific region. Dig Endosc. 2013; 25(Suppl 2):146–150. PMID: 23617667.

Fig. 2
The Prague classification of Barrett esophagus. The Prague C&M classification uses the C value for the circumferential pattern (C) and the M value for the maximum length (M) (including tongue-like pattern).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download