Abstract
Irritable bowel syndrome (IBS) is a gastrointestinal disorder characterized by chronic abdominal pain and altered bowel habits in the absence of any organic cause. As the clinical manifestations are very diverse and associated with nonspecific symptoms, research seeking to identify organic causes to rule out IBS and to enable differential diagnosis is required. A 24-year-old man was referred to our hospital for specialized management of IBS. He had a 7-month history of intermittent epigastric and lower abdominal pain. On the basis of clinical examination, he was diagnosed with IBS and administered medication at a primary clinic. However, his symptoms did not improve after treatment. We performed capsule endoscopy at our hospital and identified a parasite (Ancylostoma duodenale) in the proximal jejunum. We therefore report a case of parasitic infection found by additional examination while evaluating symptoms associated with a previous diagnosis of refractory IBS.
Irritable bowel syndrome (IBS) is defined as a gastrointestinal disorder characterized by chronic abdominal pain and altered bowel habits in the absence of any organic cause. The incidence of IBS has been reported to be approximately 12% to 22% worldwide.1-4 Although the precise pathophysiology of IBS is unknown, changes in bowel movement patterns and gut hypersensitivity have been associated with IBS. Intestinal inflammation, changes in normal intestinal bacterial flora, bacterial overgrowth in the intestine, hypersensitivity to food, and immunologic or genetic factors have been proposed as possible factors.3,5 The clinical manifestations of IBS are very diverse, including gastrointestinal symptoms and extraintestinal symptoms.4,6-8 As the symptoms are variable and nonspecific, efforts should be made to determine an organic cause of symptoms to rule out IBS and make a differential diagnosis by reviewing the patient's history, physical examinations, and additional medical examinations.9,10 We report a case of parasitic infection diagnosed by capsule endoscopy performed for the evaluation of associated symptoms in a patient previously diagnosed with refractory IBS.
A 24-year-old man was referred to our gastroenterology department for the specialized management of persistent symptoms such as intermittent epigastric and lower abdominal pain that had begun approximately 7 months earlier. The pain had developed gradually and tended to aggravate after meals.
One month prior to his referral to our department, the patient had visited a primary outpatient clinic several times and was administered medication to control symptoms, but his symptoms did not improve. The patient was later admitted to a primary hospital for 1 week, where gastroduodenoscopy, colonoscopy, abdominal sonography, and laboratory testing was performed to identify the cause of the symptoms. On the basis of these examinations, he was diagnosed with IBS and administered medication. However, because of poor symptom control, the patient was referred to our hospital for specialized management of IBS. The patient had no medical history, including drug history, and had lost approximately 5 kg over the previous 7 months. The patient had a history of 5 pack-years of smoking and social alcohol consumption. The patient's father was diagnosed with pulmonary tuberculosis 1 month earlier and was receiving medication. The patient was employed as a cell phone salesman, but helped with work on his father's farm every weekend. His associated symptoms were frequent nausea with vomiting and diarrhea-like loose stools.
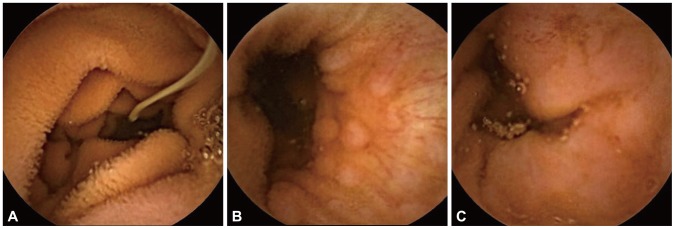
Physical examination findings were nonspecific and he showed chronic rather than acute illness. He had no tenderness or rebound tenderness on his abdomen. Laboratory test results of peripheral blood, blood biochemistry, and serum electrolytes were all within the normal ranges. Urine analysis and stool occult blood test showed no abnormal findings. Because of his weight loss, we tested his glucose and thyroid-associated hormone levels. His fasting blood glucose level was 87 mg/dL and the results of the thyroid function test were normal. We reviewed the imaging studies performed at the primary hospital. Gastroduodenoscopy showed chronic atrophic gastritis with erosion, and colonoscopy showed nonspecific terminal ileitis. Abdominal ultrasonography findings were nonspecific. After referral to our hospital, we changed his drugs to control symptoms. As intermittent lower abdominal pain persisted, we considered additional examinations such as small intestine endoscopy or capsule endoscopy to evaluate the small intestine. Considering the patient's compliance and the possibility of observation of the terminal ileum, we chose capsule endoscopy. On capsule endoscopy, the terminal ileum was normal, but a whitish tubular organism suspicious of a parasite with nearby multiple erosive lesions was observed at the proximal jejunum (Fig. 1, Supplementary Video 1 [available online at http://www.e-ce.org/]). Although we had considered the diagnosis to be IBS until that point, we changed the diagnosis to parasitic intestinal infection on the basis of the capsule endoscopic images. Additional examinations for parasitic infections, including blood testing and stool examination, were performed. His eosinophil count was 120/mm3 (reference range, 50 to 500); the results of the enzyme-linked immunosorbent assay were negative for cysticercosis, Sparganum, Paragonimus, and Clonorchis sinensis; and the result of a stool parasite test was also negative. Although the additional tests did not give additional information, based on symptoms, the results of examinations, and factors such as location (proximal jejunum), morphology (whitish small tubular shape), size (<2 cm), and erosive mucosal lesions around the organism, we diagnosed the patient with parasitic infection, and more precisely hook worm (Ancylostoma duodenale) infection at the proximal jejunum. We therefore empirically prescribed albendazole (400 mg/day) for 3 days. Ten days after administration of the drugs, the patient's symptoms slowly abated, and the patient recovered with no specific symptoms.
IBS is a relatively common disease and accounts for 12% of all visits to primary care physicians and up to 40% to 50% of all outpatient referrals to a gastroenterologist.3,11 Establishing a final diagnosis of IBS is difficult because of the various associated symptoms of IBS and the difficulty in differentially diagnosing this condition from other diseases that have symptoms similar to those of IBS.
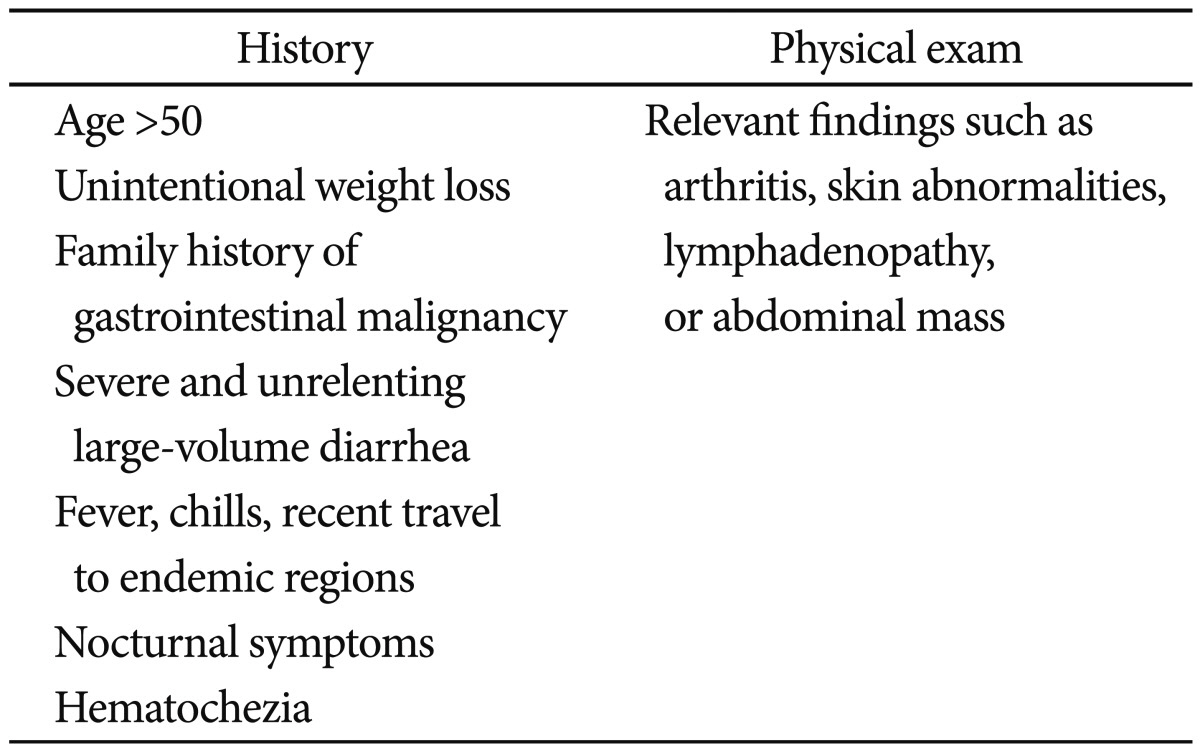
Two opposing approaches can be used in establishing a diagnosis of IBS. One approach is to view IBS as a diagnosis of exclusion and the other is to view IBS as a symptomatic condition of its own.10 As extensive diagnostic testing and invasive investigations to exclude possible organic causes are needed for the former method, the latter method, the symptom-based approach, has been broadly accepted. The Manning criteria have been used as representative criteria for diagnosis, but the Rome III criteria have also been used recently as the main criteria for the diagnosis of IBS.10,12,13 However, with symptom-based criteria, some clinicians may not make an effort to exclude other diseases through testing and investigation. Therefore, a review of warning signs should be performed before diagnosing IBS (Table 1). If the patient does exhibit warning signs, further evaluation should be undertaken. 9,14 We further evaluated a patient previously diagnosed with refractory IBS and found evidence of parasitic infection. Furthermore, after treatment of the parasitic infection, IBS could be ruled out.
The clinical manifestations of parasitic infection are similar to the symptoms of IBS, and the A. duodenale infection in this case exhibited gastrointestinal symptoms similar to those of IBS. Two species of hookworm cause human infection: A. duodenale and Necator americanus. Hookworms are so named because of their hook-like features.15 The main site of parasitic infection is the jejunum; male hookworms are 8 to 10×0.4 to 0.5 mm and female hookworms are 10 to 13×0.6 mm in size.16
A. duodenale is gray-whitish, and has more hook-like features and is longer and thicker than N. americanus.17 The principle model of transmission is contact of human skin with human fecal contaminated soil.18 Clinical manifestations include cutaneous symptoms, transpulmonary passage symptoms, and gastrointestinal symptoms such as nausea, vomiting, diarrhea, and postprandial accentuated midepigastric pain. A diagnosis of hookworm infection includes clinical manifestations, a history of skin exposure to potentially contaminated soil, and stool examination, which is the primary established tool. Effective anthelminthic treatment of hookworm infection consists of albendazole (400 mg once daily) or mebendazole (100 mg twice daily) for 3 days.15,18,19
In this case, although results for both stool egg testing and tissue confirmation of parasite infection were negative, a final diagnosis of parasite infection was established based on capsule endoscopic images, history, symptoms, and symptom improvement after anti-parasite treatment. In particular, it is important to distinguish hookworms from tapeworms (Taenia solium, Diphyllobothrium), which parasitize the small bowel. We ruled out tapeworm infection because the incidence of tapeworm infection is much rarer than hookworm infection, tapeworms are flatter and longer than hookworms, and we achieved successful treatment by the empirical administration of albendazole (whereas distocide is the treatment of choice of tapeworms).
In this paper, we present a unique case of diagnosing parasitic infection and simultaneously ruling out a previous diagnosis of refractory IBS using capsular endoscopy and relieving symptoms by the administration of the antiparasitic drug albendazole, which is broadly used for the treatment of many parasitic infections.
In conclusion, clinical physicians should be aware of the importance of ruling out any organic causes inducing IBS-like symptoms in patients with suspected IBS, especially those with refractory symptoms even with specialized management of IBS. In addition, although parasitic infection is very rare, additional examination should be performed if parasitic infection is suspected. Because there are some limitations of capsule endoscopy and small bowel endoscopy, empirical administration of antiparasitic drugs (with consideration of adverse drug effects) should be considered in uncertain or refractory IBS patients if the patient has any other evidence suspicious of parasitic infection, such as anemia, weight loss, or environmental factors that could expose the patient to infection by parasites. In addition, although it is often difficult to confirm diagnosis, we should strive to test for worms or eggs in stools if parasite infection is suspected.
References
1. Morgan DR, Benshoff M, Cáceres M, et al. Irritable bowel syndrome and gastrointestinal parasite infection in a developing nation environment. Gastroenterol Res Pract. 2012; 2012:343812. PMID: 22474433.

2. Pokkunuri V, Pimentel M, Morales W, et al. Role of cytolethal distending toxin in altered stool form and bowel phenotypes in a rat model of post-infectious irritable bowel syndrome. J Neurogastroenterol Motil. 2012; 18:434–442. PMID: 23106005.

3. Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007; 26:535–544. PMID: 17661757.

4. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006; 130:1480–1491. PMID: 16678561.

5. Spiller R, Lam C. An update on post-infectious irritable bowel syndrome: role of genetics, immune activation, serotonin and altered microbiome. J Neurogastroenterol Motil. 2012; 18:258–268. PMID: 22837873.

6. Swarbrick ET, Hegarty JE, Bat L, Williams CB, Dawson AM. Site of pain from the irritable bowel. Lancet. 1980; 2:443–446. PMID: 6106097.

7. Whorwell PJ, McCallum M, Creed FH, Roberts CT. Non-colonic features of irritable bowel syndrome. Gut. 1986; 27:37–40. PMID: 3949235.

8. Hershfield NB. Nongastrointestinal symptoms of irritable bowel syndrome: an office-based clinical survey. Can J Gastroenterol. 2005; 19:231–234. PMID: 15861265.

9. De Giorgio R, Barbara G, Stanghellini V, et al. Diagnosis and therapy of irritable bowel syndrome. Aliment Pharmacol Ther. 2004; 20(Suppl 2):10–22. PMID: 15335409.

10. Jellema P, van der Windt DA, Schellevis FG, van der Horst HE. Systematic review: accuracy of symptom-based criteria for diagnosis of irritable bowel syndrome in primary care. Aliment Pharmacol Ther. 2009; 30:695–706. PMID: 19575763.

11. Ramirez-Miranda ME, Hernandez-Castellanos R, Lopez-Escamilla E, et al. Parasites in Mexican patients with irritable bowel syndrome: a case-control study. Parasit Vectors. 2010; 3:96. PMID: 20942938.

12. Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006; 130:1377–1390. PMID: 16678553.

13. Vanner SJ, Depew WT, Paterson WG, et al. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol. 1999; 94:2912–2917. PMID: 10520844.

14. Cash BD, Chey WD. Irritable bowel syndrome: an evidence-based approach to diagnosis. Aliment Pharmacol Ther. 2004; 19:1235–1245. PMID: 15191504.
15. de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003; 19:547–551. PMID: 14642761.

16. Haas W, Haberl B, Syafruddin , et al. Behavioural strategies used by the hookworms Necator americanus and Ancylostoma duodenale to find, recognize and invade the human host. Parasitol Res. 2005; 95:30–39. PMID: 15614587.

17. Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004; 351:799–807. PMID: 15317893.

18. Gilles HM. Selective primary health care: strategies for control of disease in the developing world. XVII. Hookworm infection and anemia. Rev Infect Dis. 1985; 7:111–118. PMID: 3983524.

19. Shale M, Travis SP. Exposure to hookworms in patients with Crohn's disease. Aliment Pharmacol Ther. 2011; 34:1248–1249. PMID: 22004256.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download