1. Sivak MV. Gastrointestinal endoscopy: past and future. Gut. 2006; 55:1061–1064. PMID:
16849338.

2. Cotton PB, Barkun A, Ginsberg G, et al. Diagnostic endoscopy: 2020 vision. Gastrointest Endosc. 2006; 64:395–398. PMID:
16923489.

3. Uedo N, Iishi H, Tatsuta M, et al. A novel videoendoscopy system by using autofluorescence and reflectance imaging for diagnosis of esophagogastric cancers. Gastrointest Endosc. 2005; 62:521–528. PMID:
16185965.

4. Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004; 9:568–577. PMID:
15189095.

5. Jung SW, Lim KS, Lim JU, et al. Flexible spectral imaging color enhancement (FICE) is useful to discriminate among non-neoplastic lesion, adenoma, and cancer of stomach. Dig Dis Sci. 2011; 56:2879–2886. PMID:
21800158.

6. Hong SN, Choe WH, Lee JH, et al. Prospective, randomized, back-to-back trial evaluating the usefulness of i-SCAN in screening colonoscopy. Gastrointest Endosc. 2012; 75:1011–1021. PMID:
22381530.

7. Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004; 127:706–713. PMID:
15362025.

8. Tajiri H, Niwa H. Proposal for a consensus terminology in endoscopy: how should different endoscopic imaging techniques be grouped and defined? Endoscopy. 2008; 40:775–778. PMID:
18698532.

9. Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001; 219:316–333. PMID:
11323453.

10. Thakur M, Lentle BC. Report of a summit on molecular imaging. Radiology. 2005; 236:753–755. PMID:
16118158.

11. Mahmood U, Wallace MB. Molecular imaging in gastrointestinal disease. Gastroenterology. 2007; 132:11–14. PMID:
17241854.

12. Takayama T, Katsuki S, Takahashi Y, et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med. 1998; 339:1277–1284. PMID:
9791143.

13. Keller R, Winde G, Eisenhawer C, et al. Immunoscopy: a technique combining endoscopy and immunofluorescence for diagnosis of colorectal carcinoma. Gastrointest Endosc. 1998; 47:154–161. PMID:
9512281.
14. Pasricha PJ, Motamedi M. Optical biopsies, "bioendoscopy," and why the sky is blue: the coming revolution in gastrointestinal imaging. Gastroenterology. 2002; 122:571–575. PMID:
11832471.

15. Fujimoto JG, Brezinski ME, Tearney GJ, et al. Optical biopsy and imaging using optical coherence tomography. Nat Med. 1995; 1:970–972. PMID:
7585229.

16. Weissleder R. Molecular imaging in cancer. Science. 2006; 312:1168–1171. PMID:
16728630.

17. Goetz M, Hoetker MS, Diken M, Galle PR, Kiesslich R. In vivo molecular imaging with cetuximab, an anti-EGFR antibody, for prediction of response in xenograft models of human colorectal cancer. Endoscopy. 2013; 45:469–477. PMID:
23580409.

18. Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011; 17:1685–1691. PMID:
22057348.

19. Keller R, Winde G, Terpe HJ, Foerster EC, Domschke W. Fluorescence endoscopy using a fluorescein-labeled monoclonal antibody against carcinoembryonic antigen in patients with colorectal carcinoma and adenoma. Endoscopy. 2002; 34:801–807. PMID:
12244502.

20. Marten K, Bremer C, Khazaie K, et al. Detection of dysplastic intestinal adenomas using enzyme-sensing molecular beacons in mice. Gastroenterology. 2002; 122:406–414. PMID:
11832455.

21. Hsiung PL, Hardy J, Friedland S, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med. 2008; 14:454–458. PMID:
18345013.

22. Ito S, Muguruma N, Kusaka Y, et al. Detection of human gastric cancer in resected specimens using a novel infrared fluorescent anti-human carcinoembryonic antigen antibody with an infrared fluorescence endoscope in vitro. Endoscopy. 2001; 33:849–853. PMID:
11571680.

23. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science. 2013; 339:1546–1558. PMID:
23539594.

24. Funovics MA, Alencar H, Montet X, Weissleder R, Mahmood U. Simultaneous fluorescence imaging of protease expression and vascularity during murine colonoscopy for colonic lesion characterization. Gastrointest Endosc. 2006; 64:589–597. PMID:
16996355.
25. Petrovsky A, Schellenberger E, Josephson L, Weissleder R, Bogdanov A Jr. Near-infrared fluorescent imaging of tumor apoptosis. Cancer Res. 2003; 63:1936–1942. PMID:
12702586.
26. Citrin D, Lee AK, Scott T, et al. In vivo tumor imaging in mice with near-infrared labeled endostatin. Mol Cancer Ther. 2004; 3:481–488. PMID:
15078992.
27. Hoetker MS, Kiesslich R, Diken M, et al. Molecular in vivo imaging of gastric cancer in a human-murine xenograft model: targeting epidermal growth factor receptor. Gastrointest Endosc. 2012; 76:612–620. PMID:
22771099.

28. Goetz M, Ziebart A, Foersch S, et al. In vivo molecular imaging of colorectal cancer with confocal endomicroscopy by targeting epidermal growth factor receptor. Gastroenterology. 2010; 138:435–446. PMID:
19852961.

29. Foersch S, Kiesslich R, Waldner MJ, et al. Molecular imaging of VEGF in gastrointestinal cancer in vivo using confocal laser endomicroscopy. Gut. 2010; 59:1046–1055. PMID:
20639250.

30. Bando T, Muguruma N, Ito S, et al. Basic studies on a labeled anti-mucin antibody detectable by infrared-fluorescence endoscopy. J Gastroenterol. 2002; 37:260–269. PMID:
11993509.

31. Rudin M, Weissleder R. Molecular imaging in drug discovery and development. Nat Rev Drug Discov. 2003; 2:123–131. PMID:
12563303.

32. Goetz M, Wang TD. Molecular imaging in gastrointestinal endoscopy. Gastroenterology. 2010; 138:828–833. PMID:
20096697.

33. Kelly K, Alencar H, Funovics M, Mahmood U, Weissleder R. Detection of invasive colon cancer using a novel, targeted, library-derived fluorescent peptide. Cancer Res. 2004; 64:6247–6251. PMID:
15342411.

34. Fujikawa Y, Urano Y, Komatsu T, et al. Design and synthesis of highly sensitive fluorogenic substrates for glutathione S-transferase and application for activity imaging in living cells. J Am Chem Soc. 2008; 130:14533–14543. PMID:
18841967.

35. Joshi BP, Liu Z, Elahi SF, Appelman HD, Wang TD. Near-infrared-labeled peptide multimer functions as phage mimic for high affinity, specific targeting of colonic adenomas in vivo (with videos). Gastrointest Endosc. 2012; 76:1197–1206. PMID:
23022051.

36. Wu X, Liu H, Liu J, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003; 21:41–46. PMID:
12459735.

37. Mordon S, Devoisselle JM, Soulie-Begu S, Desmettre T. Indocyanine green: physicochemical factors affecting its fluorescence in vivo. Microvasc Res. 1998; 55:146–152. PMID:
9521889.
38. Ogawa M, Kosaka N, Choyke PL, Kobayashi H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res. 2009; 69:1268–1272. PMID:
19176373.

39. Nussbaum S, Roth HJ. Human anti-mouse antibodies: pitfalls in tumor marker measurement and strategies for enhanced assay robustness: including results with Elecsys CEA. Anticancer Res. 2000; 20(6D):5249–5252. PMID:
11326704.
40. Bird-Lieberman EL, Neves AA, Lao-Sirieix P, et al. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett's esophagus. Nat Med. 2012; 18:315–321. PMID:
22245781.

41. Ozdemir V, Williams-Jones B, Glatt SJ, Tsuang MT, Lohr JB, Reist C. Shifting emphasis from pharmacogenomics to theragnostics. Nat Biotechnol. 2006; 24:942–946. PMID:
16900136.

42. Weissleder R. Molecular imaging: exploring the next frontier. Radiology. 1999; 212:609–614. PMID:
10478223.

43. Barrett T, Koyama Y, Hama Y, et al. In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin Cancer Res. 2007; 13(22 Pt 1):6639–6648. PMID:
17982120.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


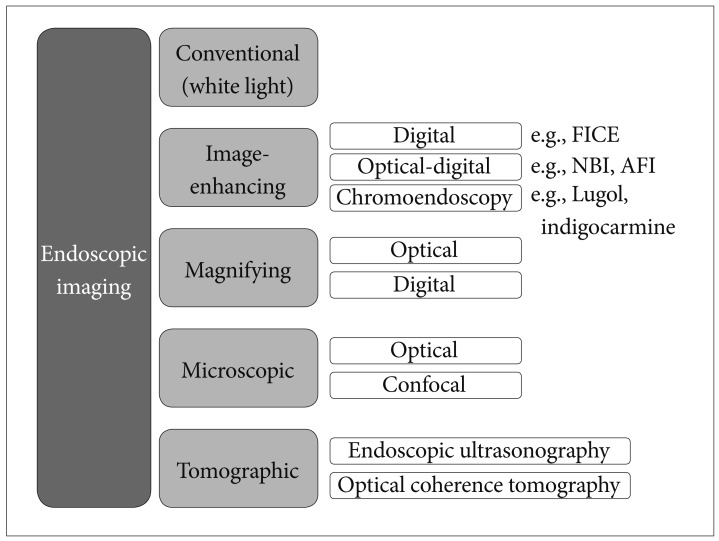
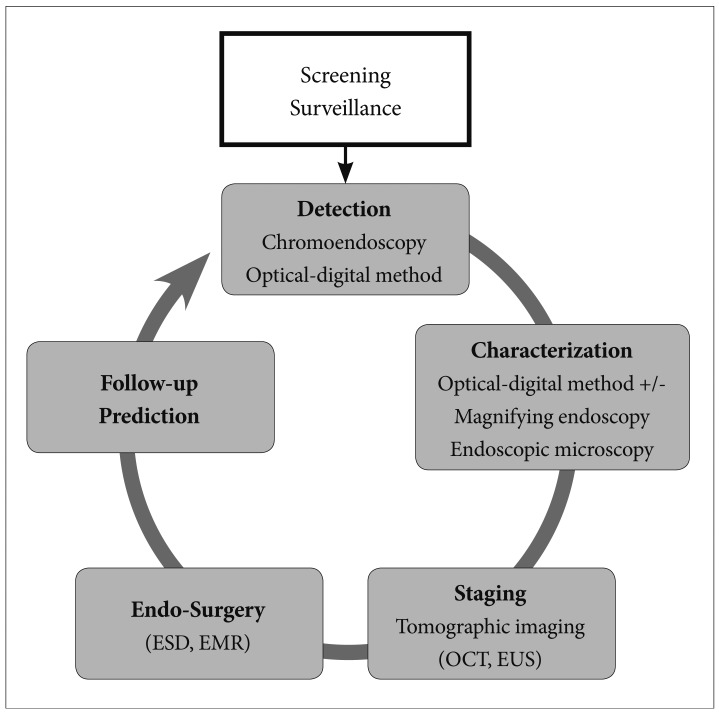
 XML Download
XML Download