1. Bakkevold KE, Arnesjø B, Dahl O, Kambestad B. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater: results of a controlled, prospective, randomised multicentre study. Eur J Cancer. 1993; 29A:698–703. PMID:
8471327.
2. Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993; 165:68–72. PMID:
8380315.

3. Jung GS, Song HY, Kang SG, et al. Malignant gastroduodenal obstructions: treatment by means of a covered expandable metallic stent-initial experience. Radiology. 2000; 216:758–763. PMID:
10966707.

4. Fujino Y, Suzuki Y, Kamigaki T, Mitsutsuji M, Kuroda Y. Evaluation of gastroenteric bypass for unresectable pancreatic cancer. Hepatogastroenterology. 2001; 48:563–568. PMID:
11379354.
5. Baron TH, Harewood GC. Enteral self-expandable stents. Gastrointest Endosc. 2003; 58:421–433. PMID:
14528223.

6. Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction. Analysis of technical and functional efficacy. Arch Intern Med. 1985; 145:1443–1446. PMID:
4026476.

7. Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002; 97:72–78. PMID:
11808972.

8. Kim JH, Song HY, Shin JH, et al. Metallic stent placement in the palliative treatment of malignant gastroduodenal obstructions: prospective evaluation of results and factors influencing outcome in 213 patients. Gastrointest Endosc. 2007; 66:256–264. PMID:
17643698.

9. Dormann A, Meisner S, Verin N, Wenk Lang A. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004; 36:543–550. PMID:
15202052.

10. Lindsay JO, Andreyev HJ, Vlavianos P, Westaby D. Self-expanding metal stents for the palliation of malignant gastroduodenal obstruction in patients unsuitable for surgical bypass. Aliment Pharmacol Ther. 2004; 19:901–905. PMID:
15080851.

11. Sabharwal T, Irani FG, Adam A. Cardiovascular and Interventional Radiological Society of Europe. Quality assurance guidelines for placement of gastroduodenal stents. Cardiovasc Intervent Radiol. 2007; 30:1–5. PMID:
17103108.

12. Bessoud B, de Baere T, Denys A, et al. Malignant gastroduodenal obstruction: palliation with self-expanding metallic stents. J Vasc Interv Radiol. 2005; 16(2 Pt 1):247–253. PMID:
15713926.

13. Song HY, Yang DH, Kuh JH, Choi KC. Obstructing cancer of the gastric antrum: palliative treatment with covered metallic stents. Radiology. 1993; 187:357–358. PMID:
7682722.

14. Lopera JE, Brazzini A, Gonzales A, Castaneda-Zuniga WR. Gastroduodenal stent placement: current status. Radiographics. 2004; 24:1561–1573. PMID:
15537965.

15. Mauro MA, Koehler RE, Baron TH. Advances in gastrointestinal intervention: the treatment of gastroduodenal and colorectal obstructions with metallic stents. Radiology. 2000; 215:659–669. PMID:
10831681.

16. Piesman M, Kozarek RA, Brandabur JJ, et al. Improved oral intake after palliative duodenal stenting for malignant obstruction: a prospective multicenter clinical trial. Am J Gastroenterol. 2009; 104:2404–2411. PMID:
19707192.

17. Cho YK, Kim SW, Hur WH, et al. Clinical outcomes of self-expandable metal stent and prognostic factors for stent patency in gastric outlet obstruction caused by gastric cancer. Dig Dis Sci. 2010; 55:668–674. PMID:
19333756.

18. Telford JJ, Carr-Locke DL, Baron TH, et al. Palliation of patients with malignant gastric outlet obstruction with the enteral Wallstent: outcomes from a multicenter study. Gastrointest Endosc. 2004; 60:916–920. PMID:
15605006.

19. Kim JH, Song HY, Shin JH, et al. Metallic stent placement in the palliative treatment of malignant gastric outlet obstructions: primary gastric carcinoma versus pancreatic carcinoma. AJR Am J Roentgenol. 2009; 193:241–247. PMID:
19542420.

20. Song HY, Kim TH, Choi EK, et al. Metallic stent placement in patients with recurrent cancer after gastrojejunostomy. J Vasc Interv Radiol. 2007; 18:1538–1546. PMID:
18057289.

21. Maire F, Hammel P, Ponsot P, et al. Long-term outcome of biliary and duodenal stents in palliative treatment of patients with unresectable adenocarcinoma of the head of pancreas. Am J Gastroenterol. 2006; 101:735–742. PMID:
16635221.

22. Jung GS, Song HY, Seo TS, et al. Malignant gastric outlet obstructions: treatment by means of coaxial placement of uncovered and covered expandable nitinol stents. J Vasc Interv Radiol. 2002; 13:275–283. PMID:
11875087.

23. Gaidos JK, Draganov PV. Treatment of malignant gastric outlet obstruction with endoscopically placed self-expandable metal stents. World J Gastroenterol. 2009; 15:4365–4371. PMID:
19764086.

24. Nassif T, Prat F, Meduri B, et al. Endoscopic palliation of malignant gastric outlet obstruction using self-expandable metallic stents: results of a multicenter study. Endoscopy. 2003; 35:483–489. PMID:
12783345.

25. Masci E, Viale E, Mangiavillano B, et al. Enteral self-expandable metal stent for malignant luminal obstruction of the upper and lower gastrointestinal tract: a prospective multicentric study. J Clin Gastroenterol. 2008; 42:389–394. PMID:
18277900.
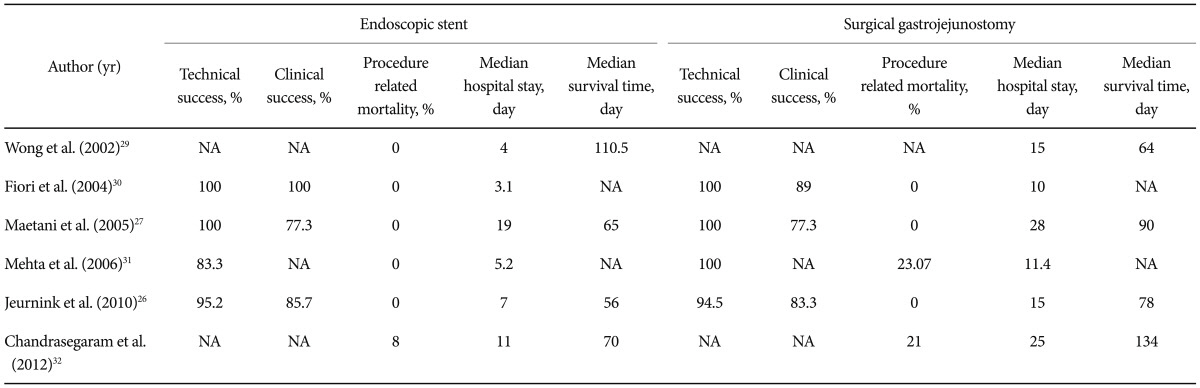
26. Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010; 71:490–499. PMID:
20003966.

27. Maetani I, Tada T, Ukita T, Inoue H, Sakai Y, Nagao J. Comparison of duodenal stent placement with surgical gastrojejunostomy for palliation in patients with duodenal obstructions caused by pancreaticobiliary malignancies. Endoscopy. 2004; 36:73–78. PMID:
14722859.

28. Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol. 2007; 42:283–290. PMID:
17464457.

29. Wong YT, Brams DM, Munson L, et al. Gastric outlet obstruction secondary to pancreatic cancer: surgical vs endoscopic palliation. Surg Endosc. 2002; 16:310–312. PMID:
11967685.
30. Fiori E, Lamazza A, Volpino P, et al. Palliative management of malignant antro-pyloric strictures. Gastroenterostomy vs. endoscopic stenting. A randomized prospective trial. Anticancer Res. 2004; 24:269–271. PMID:
15015607.
31. Mehta S, Hindmarsh A, Cheong E, et al. Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg Endosc. 2006; 20:239–242. PMID:
16362479.

32. Chandrasegaram MD, Eslick GD, Mansfield CO, et al. Endoscopic stenting versus operative gastrojejunostomy for malignant gastric outlet obstruction. Surg Endosc. 2012; 26:323–329. PMID:
21898024.

33. Kaw M, Singh S, Gagneja H, Azad P. Role of self-expandable metal stents in the palliation of malignant duodenal obstruction. Surg Endosc. 2003; 17:646–650. PMID:
12404051.

34. Mutignani M, Tringali A, Shah SG, et al. Combined endoscopic stent insertion in malignant biliary and duodenal obstruction. Endoscopy. 2007; 39:440–447. PMID:
17516351.
35. van Hooft JE, Uitdehaag MJ, Bruno MJ, et al. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest Endosc. 2009; 69:1059–1066. PMID:
19152912.

36. Kim TO, Kang DH, Kim GH, et al. Self-expandable metallic stents for palliation of patients with malignant gastric outlet obstruction caused by stomach cancer. World J Gastroenterol. 2007; 13:916–920. PMID:
17352023.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download