Abstract
Esophageal carcinoid tumors remain some of the rarest of all carcinoid tumors, with only several cases previously reported in the literature. The endoscopic mucosal resection of selected carcinoid tumors has been shown to be a valid, safe, and effective method of treatment. Endoscopic ultrasonography is the technique of choice to select patients eligible for endoscopic resection. Here, we report successful endoscopic mucosal resection of a low esophageal carcinoid tumor and review the relevant literature. The present case is the first reported case of esophageal carcinoid tumor in Korea.
In 1969, Brenner et al.1 were the first to describe a case of carcinoid tumor originating within the esophagus. Esophageal carcinoid tumors are thought to arise from the argyrophilic endocrine cells of the mucosa, which were first detected in 1974.2 Esophageal carcinoid tumors remain some of the rarest of all carcinoid tumors, with only several cases previously reported in the literature. Here, we report successful endoscopic mucosal resection (EMR) of a low esophageal carcinoid tumor and review the literature. To our knowledge, there have been no previous case reports of esophageal carcinoid tumors in Korea.
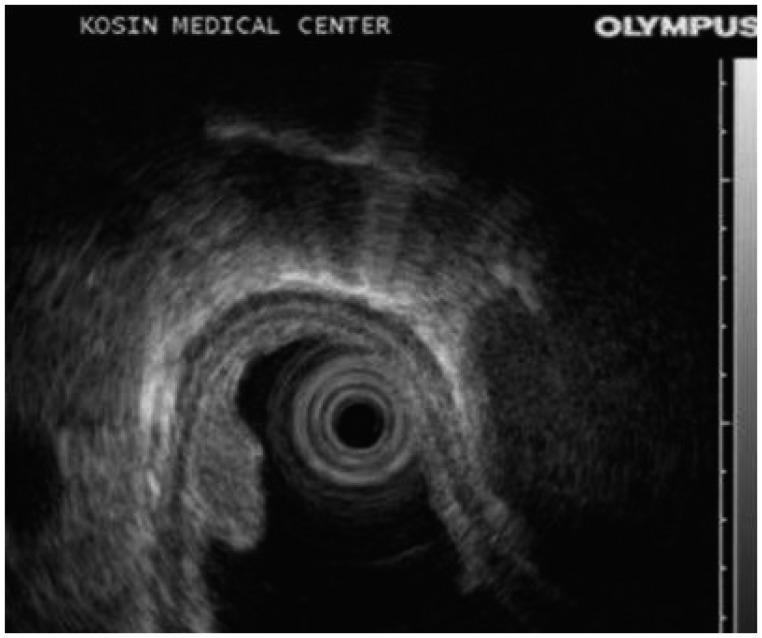
A 49-year-old woman underwent upper endoscopy due to intermittent epigastric soreness and heartburn. A 0.8×0.5-cm, ovoid, elevated, submucosal esophageal lesion was detected 35 cm from the incisors (Fig. 1). The overlying mucosa was intact but showed slightly erythematous central change and had a soft consistency. The surface of the tumor was stained with iodine. Endoscopic ultrasonography (EUS; 20 MHz, miniprobe) demonstrated a 4.4×3.3-mm, well demarcated, homogenous, hypoechoic, round mass lesion within the mucosal layer, and the submucosal layer beneath the lesion was observed to be intact (Fig. 2).
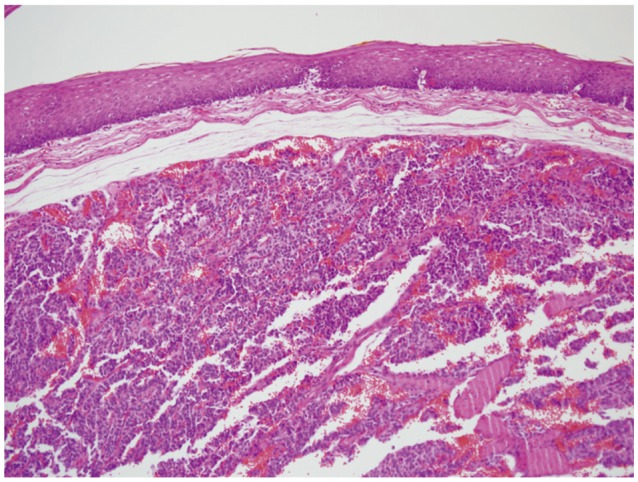
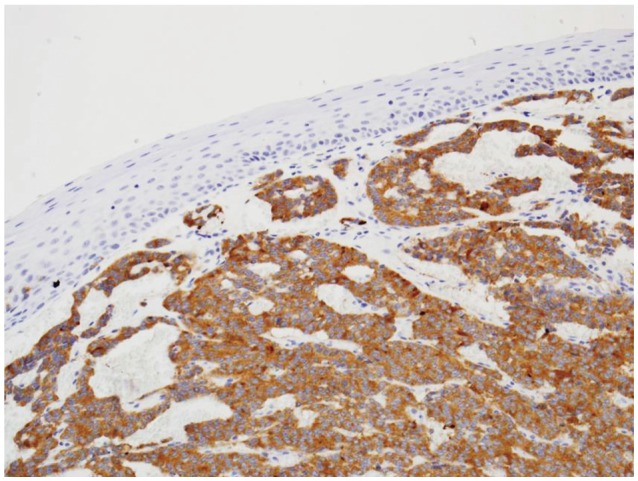
A biopsy was performed and the mass was diagnosed as a carcinoid tumor. We informed the patient of the characteristics of carcinoid tumor. The patient chose to undergo endoscopic resection. EMR was performed by snaring the lesion following a submucosal saline injection (Fig. 3). There were no immediate complications. Histologic examination of the resected specimen revealed that the tumor was located in the mucosal layer (between the mucosa and muscularis mucosa) as indicated by EUS. The tumor consisted of round cells arranged in sheets without mitoses, and the resection margin was free (Fig. 4). Immunohistochemical studies demonstrated positivity for neuron-specific enolase, synaptophysin and chromogranin as supporting evidence of an esophageal carcinoid tumor (Fig. 5). A second endoscopy performed 2 months after the first demonstrated a linear whitish scar, with no residual lesion. Computed tomography imaging of the thorax was unremarkable.
In the present patient, there were no such clinical manifestations.
Lindberg et al.6 reported that esophageal carcinoid tumors exhibit marked male predominance, with a male to female ratio of 6:1. Age at diagnosis is widely variable, ranging from 45 to 82 years. Symptoms presented in their study were dysphagia (64%), weight loss (43%), pain (14%), reflux esophagitis (14%), fatigue (7%), and melanotic stools (7%).6 Only one patient had previously reported symptoms consistent with carcinoid syndrome.7
Klöppel et al.8 reported that most esophageal carcinoid tumors histopathologically are poorly differentiated neuroendocrine carcinomas and mixed endocrine-exocrine carcinomas, usually ranging from 4 to 10 cm in diameter. They present as fungating or ulcerated masses deeply infiltrating into the esophageal wall and are associated with early spread to the regional lymph nodes or infiltration of adjacent organs. In contrast, most well-differentiated neuroendocrine tumor/neuroendocrine carcinomas are less than 4 cm in diameter, present as polypoid lesions and are rarely associated with lymph node metastases and thus have an excellent prognosis.8
The management of locoregional carcinoid tumors depends on tumor site and primary site as well as the general condition of the patient. Resection is the primary treatment approach for most localized carcinoid tumor.9 Most early reports recommend that esophageal carcinoid tumors be treated surgically by esophagogastrectomy or subtotal esophagectomy with gastroesophageal anastomosis.1,10,11 Currently, however, EMR of selected carcinoid tumors is accepted as a safe and effective alternative.12 The use of a ligation band at the lesion base may increase the ease and safety of EMR, and therefore ligation bands have been utilized for the treatment of other esophageal pathologies (i.e., Barrett's dysplasia) and for rectal carcinoid resection.13
EUS allows accurate description of the depth of tumor invasion into the esophageal wall, as well as the identification of internal features of the tumor.14 In our patient, the location of the tumor was clearly demonstrated by EUS and was consistent with pathological findings. EUS appears to be an excellent method to detect the depth of tumor invasion in the esophageal wall and has proven utility in staging and interventional planning (endoscopic or surgical) for gastrointestinal carcinoid tumors.15
Esophageal carcinoid tumors is a very rare disease. However, with increasing rates of screening upper endoscopy and recent technical improvement in endoscopy and radiology, detection of small esophageal carcinoid tumors may be increasing, adding to our findings. Due to improvements in EUS imaging techniques and EMR techniques, endoscopic treatment can successfully achieve complete resection of selected esophageal carcinoid tumors with few complications. We report a case of successful EMR of a low esophageal carcinoid tumor.
References
1. Brenner S, Heimlich H, Widman M. Carcinoid of esophagus. N Y State J Med. 1969; 69:1337–1339. PMID: 5255420.
2. Tateishi R, Taniguchi H, Wada A, Horai T, Taniguchi K. Argyrophil cells and melanocytes in esophageal mucosa. Arch Pathol. 1974; 98:87–89. PMID: 4134908.
3. Capella C, Solcia E, Sobin LH, Arnold R. Endocrine tumours of the oesophagus. In : Hamilton SR, Aaltonen LA, editors. Pathology and Gen etics of Tumours of the Digestive System. Lyon: International Agency for Research on Cancer;2000. p. 26–27.
4. Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997; 79:813–829. PMID: 9024720.

5. Modlin IM, Shapiro MD, Kidd M. An analysis of rare carcinoid tumors: clarifying these clinical conundrums. World J Surg. 2005; 29:92–101. PMID: 15599742.

6. Lindberg GM, Molberg KH, Vuitch MF, Albores-Saavedra J. Atypical carcinoid of the esophagus: a case report and review of the literature. Cancer. 1997; 79:1476–1481. PMID: 9118026.
7. Broicher K, Hienz HA. Carcinoid syndrome in primary esophageal tu mor. Z Gastroenterol. 1974; 12(Suppl):377–384. PMID: 4418379.
8. Klöppel G, Rindi G, Anlauf M, Perren A, Komminoth P. Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Arch. 2007; 451(Suppl 1):S9–S27. PMID: 17684761.

9. Kulke MH, Benson AB 3rd, Bergsland E, et al. Neuroendocrine tumors. J Natl Compr Canc Netw. 2012; 10:724–764. PMID: 22679117.
10. Chong FK, Graham JH, Madoff IM. Mucin-producing carcinoid ("composite tumor") of upper third of esophagus: a variant of carcinoid tu mor. Cancer. 1979; 44:1853–1859. PMID: 227580.
11. Younghusband JD, Aluwihare AP. Carcinoma of the oesophagus: factors influencing survival. Br J Surg. 1970; 57:422–430. PMID: 5431572.

12. Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002; 55:390–396. PMID: 11868015.

13. Mashimo Y, Matsuda T, Uraoka T, et al. Endoscopic submucosal resection with a ligation device is an effective and safe treatment for carcino id tumors in the lower rectum. J Gastroenterol Hepatol. 2008; 23:218–221. PMID: 18289355.
14. Akasu T, Moriya Y, Sugihara K. Transrectal ultrasonography of a small rectal carcinoid tumor with lymph node metastasis: a case report. Jpn J Clin Oncol. 1996; 26:112–115. PMID: 8609694.

15. Varas Lorenzo MJ, Miquel Collell JM, Maluenda Colomer MD, Boix Valverde J, Armengol Miro JR. Preoperative detection of gastrointestinal neuroendocrine tumors using endoscopic ultrasonography. Rev Esp Enferm Dig. 2006; 98:828–836. PMID: 17198475.

Fig. 1
Endoscopic view of the lesion. An 0.8×0.5-cm, ovoid, elevated, submucosal esophageal lesion was noted 35 cm from the upper incisors.
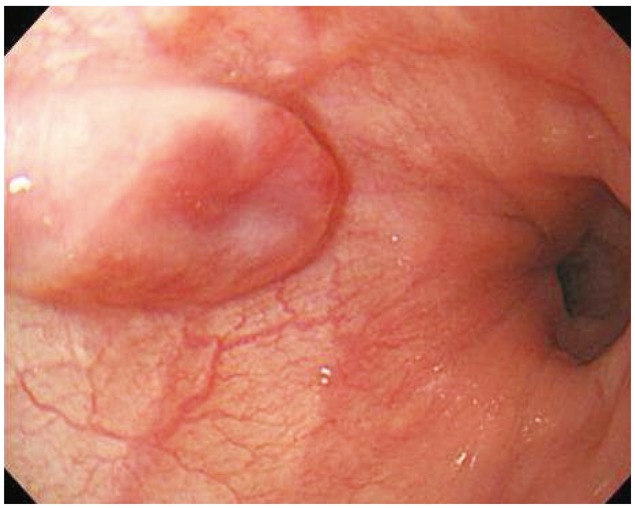
Fig. 2
Endoscopic ultrasound image of the lesion. Endoscopic ultrasonography shows homogenous, hypoechoic lesion within the mucosal layer.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download