Abstract
Currently, endoscopic retrograde cholangiopancreatography (ERCP) is the preferred procedure for biliary drainage for various pancreatico-biliary disorders. ERCP is successful in 90% of the cases, but is unsuccessful in cases with altered anatomy or with tumors obstructing access to the duodenum. Due to the morbidity and mortality associated with surgical or percutaneous approaches in unsuccessful ERCP cases, biliary endoscopists have been using endoscopic ultrasound-guided biliary drainage (EUS-BD) more frequently within the last decade in different countries. As with any novel advanced endoscopic procedure that incorporates various approaches, advanced endoscopists all over the world have innovated and adopted diverse EUS-BD techniques. Indications for EUS-BD include failed conventional ERCP, altered anatomy, tumor preventing access into the biliary tree and contraindication to percutaneous access (i.e., ascites, etc.). EUS-BD utilizing EUS-guided rendezvous technique is conducted by creating a tract from either the stomach or the duodenum into the bile duct. Although EUS-BD has rapidly been gaining attraction and popularity in the endoscopic world, the indications and methods have yet to be standardized. There are several access routes and techniques that are employed by advanced endoscopists throughout the world for BD. This article reviews the indications and currently practiced EUS-BD techniques, including indications, technical details (intrahepatic or extrahepatic approach), equipment, patient selection, complications, and overall advantages and limitations.
Currently accepted quality indicators for endoscopic retrograde cholangiopancreatography (ERCP) limit cannulation failure rates to 3% to 5% in the hands of high volume endoscopists.1 Common reasons for failed biliary cannulation at specialized centers include variant anatomy, malignant luminal or biliary obstruction, and periampullary diverticula.2,3 The conventional alternate options for management of biliary obstruction after unsuccessful ERCP include percutaneous transhepatic biliary drainage (BD) or surgery.4-6 However, the long recovery times, delays in receiving chemotherapy, and discomfort of percutaneous drains can significantly burden patients with limited life expectancies. The significant morbidity and mortality associated with these procedures motivated providers to create less invasive means to treat this patient population.
The first hint that endoscopic ultrasound (EUS)-guided BD may be a solution to the problem came in 1996 when Wiersema et al.7 published the first case of EUS-guided cholangiography. Seven years later, Giovannini et al.8 took the next step and reported the first case of EUS-BD in a patient with inoperable hilar obstruction. In 2006, in a small case series, Kahaleh et al.9 described the first rendezvous procedure using EUS as opposed to interventional radiology assistance.
Although EUS-BD has rapidly been gaining attraction and popularity in the endoscopic world, the indications and methods have yet to be standardized. A consortium of 40 expert endoscopists from around the world met in 2011 to try and standardize definitions, nomenclature, and indications.10 While the nomenclature has yet to be set, generally accepted indications for EUS-BD include: 1) failed conventional ERCP, 2) altered anatomy, 3) tumor preventing access into the biliary tree, and 4) contraindication to percutaneous access such as large ascites.
This review will summarize the principles and methods of performing EUS-BD along with the existing published literature on the topic.
Although EUS-BD is still a technique in evolution, there are some guiding principles that clarify the justification, approach, and appropriate setting for this procedure. There are multiple benefits of performing EUS-BD over percutaneous or surgical routes. One logistical and economic benefit is the ability to perform EUS-BD during the same visit as an unsuccessful ERCP without needing to reschedule a subsequent procedure. It also confers physiologic and anatomic advantages by tailoring various routes of internal BD depending on the individual's specific malignant obstruction.10
There are six possible methods of BD by EUS-BD. The first decision point is whether to gain biliary access via the intrahepatic or extrahepatic approach. Once access is acquired, biliary decompression can be achieved in either a transmural or transpapillary fashion. The transpapillary route can be further divided into antegrade or retrograde (i.e., rendezvous) drainage. The choice of approach should be individualized for each patient as there are no prospective studies showing superiority of one method over the other.
Because EUS-BD is a novel, advanced procedure, it requires special expertise to perform. A consensus at the 2011 consortium agreed that EUS-BD should be attempted by trained pancreaticobiliary endoscopists who successfully complete 200 to 300 EUS and ERCP procedures annually, meet quality indicators, and have appropriate interventional radiology and surgical backup.10
To maximize chances of success, prevent delays, and minimize complications, it is important to have a plan of approach along with all the necessary equipment easily available prior to beginning an EUS-BD procedure. After access has been gained to the biliary system, the endoscopist must be steady, focused, and deliberate in their actions to maintain wire access, minimize manipulations, and prevent complications.
1) Fluoroscopy: EUS-BD cannot be accomplished safely without fluoroscopy as it allows for visualization of the needle angle prior to duct puncture and subsequent confirmation of biliary access. The needle should enter in the bile duct in the caudad direction when attempting transpapillary drainage and in the cephalad direction during choledochoduodenostomy (CDS).
2) Contrast media: contrast used during EUS-BD is usually water-soluble, iodine-based. After needle puncture into the biliary system, contrast injection allows for confirmation of location prior to further manipulation.
3) Water to flush catheters and hydrophilic wires.
4) Echoendoscope with a 3.8 mm working channel (to avoid instrument limitation). If rendezvous procedure is being attempted, then a side-viewing duodenoscope should also be available.
5) EUS-guided fine needle aspiration (FNA) needles: most case series have reported use of a 19 gauge (G) needle for EUS-BD over the 22 G needle because of easier manipulation of a 0.035-inch guidewire.
6) Hydrophilic 0.035-inch guidewires are preferred due to the ease of their manipulation. Uncoated guidewires may be safer to prevent any shearing that the EUS-FNA needle may have on the wire.
7) Bougie catheters and dilating balloons: generally the preferred calibers are 6 to 7 Fr for the bougie catheter and 4 to 6 mm for the dilating balloons.
8) Rotating sphincterotome with bend capability can allow for redirection of the wire towards either the papilla or proximal biliary system depending on the endoscopist's preference.
9) Stents: both plastic (straight, double pigtail) or metal (covered or partially-covered) can be utilized. The evidence to guide stent selection is discussed in a later section in this article.
The intrahepatic approach usually involves gaining access to the biliary system with either a transpesophageal, transgastric, or transjejunal (in altered anatomy) needle puncture into the left hepatic system. Biliary segment III of the left hepatic lobe is usually best visualized from the stomach cardia or lesser curvature. Most endoscopists performing this procedure prefer a 19 G needled for initial entry as it allows for easier wire manipulation, while maintaining transfer of force.10 Confirmation of biliary access can be obtained by aspiration of bile and subsequent injection of contrast to perform a cholangiogram. Wire manipulation is a critical portion of EUS-BD as it dictates the subsequent route of drainage (transpapillary or transmural) and can lead to complications (shearing, perforation) if not performed in a careful manner.11 Therefore it is important to thoroughly flush the instrument channel with water to minimize friction and allow for easier passage and manipulation of the wire into the biliary system. To minimize complications, the wire should not be pulled back against resistance. Also, the puncture tract should be dilated with a bougie or balloon in cases where transpapillary positioning is challenging and ongoing wire manipulation is required.
In most cases, the goal of initial access is to allow direction of the wire in the caudad direction towards the ampulla and duodenum. If transpapillary access is obtained then BD may be performed in the antegrade or rendezvous stent placement. If transpapillary positioning is impossible, then drainage should be pursued via the transmural route, which requires fistula tract dilation with subsequent transmural stent placement.
The extrahepatic EUS-BD technique entails needle puncture from the transduodenal or transantral route directly into the common bile duct (CBD). Some advantages of the extrahepatic approach include better visualization of the CBD or common hepatic duct compared to intrahepatic segments depending on the level of the obstruction. Also because the CBD is in the retroperitoneal space, extrahepatic access may be a safer option for patients with ascites.10 One limitation of this approach however is the difficulty of antegrade stent placement due to the angle of needle entry into the biliary system. Therefore, when engaging in extrahepatic EUS-BD, the two drainage options that will be most likely successful are rendezvous or transmural (CDS). After needle puncture, the same steps outlined above can be used to confirm location and direct further wire access. Generally needle angle in the cephalad direction facilitates CDS while the caudad direction will allow for possible rendezvous.
The objective of the rendezvous approach is to perform BD with a standard side-viewing duodenoscope in the usual retrograde fashion. The distinction from standard ERCP is that the initial wire has traversed the papilla in an antegrade fashion via EUS-guidance rather than by retrograde cannulation with a duodenoscope. In cases of failed ERCP, if the duodenoscope can be advanced to the ampulla and a wire can be placed across by the papilla via EUS-guidance, this is the preferred method of BD. The limiting factor in many cases for this technique is the inability of the duodenoscope to traverse a luminal obstruction. When attempting rendezvous, the initial needle angle prior to puncture should be in the caudad direction to direct it towards the duodenum.12 Generally, this angle is most often achieved with the scope in a short position, but may require several tip deflections and scope position manuevers to optimize the needle direction. After needle puncture into the biliary system, various calibers (0.035-, 0.021-, and 0.018-inch) of long (450 cm) wire can be used to pass across the papilla into the small intestines.12 Generally passing enough wire to form loops in the duodenum, which can be confirmed under fluoroscopy, is prudent to allow enough slack to maintain wire position as the echoendoscope is removed from the patient over the wire. After the echoendoscope has been removed and the wire is successfully maintained in the transpapillary position, a standard side-viewing endoscope can be advanced to the major ampulla where the EUS-placed wire can be captured with a forceps and pulled back into the instrument channel. This allows for the endoscopist to proceed with BD in the conventional retrograde fashion, now that biliary access is secure.
In cases where transpapillary wire access is obtained with EUS-guidance, but rendezvous is not possible because of luminal obstruction, then antegrade placement of a biliary stent across an obstruction is feasible. Before deploying an antegrade stent, generally the transmural tract must be dilated with either a bougie or dilating balloon to allow for stent passage into the biliary system. The stent can be placed across the papilla itself or bridge a malignant stricture to provide an outlet for bile drainage by reducing the pressure gradient across the biliary system. This technique can be performed with or without an adjunctive transmural (i.e., hepaticogastrostomy) stent. Antegrade stent placement from the extrahepatic approach is challenging due to the angulation of the wire from this position.10
During EUS-BD, if a wire cannot be positioned across the papilla either due to difficult position or complete tumor infiltration of the bile duct, then transmural drainage should be performed to relieve the biliary obstruction. In the intrahepatic approach this requires creation of a transgastric-transhepatic fistula and in the extrahepatic approach a transenterictranscholechocal fistula.11 After accessing the biliary system via needle puncture the fistula tract should be dilated over the guidewire with a 4 to 6 mm dilating balloon or a 6 to 7 Fr bougie. In the extrahepatic approach, generally a cephalad needle entry toward the hilum (usually obtained with the echoendoscope in the long position in the proximal duodenum) promotes easier passage of the stent due to the less acute angle of entry. The objective of proper stent choice and placement are twofold: 1) to optimize BD and 2) minimized complications such as migration and contralateral luminal injury. Thus the use of uncovered metal stents is contraindicated due to the risk of bile leak and peritonitis. The choice between covered self-expanding metal stents (CSEMSs) and plastic stents is discussed later in this article.
Because EUS-BD is a novel and evolving technique, there is limited prospective data comparing efficacy and safety outcomes using various instruments such as needles, dilators, and stents. Most of the published literature is in the form of retrospective case series, thus the recommendations in this article are based primarily on anecdotal evidence and expert opinion. More studies need to be done to determine the optimal tools to ensure the best patient outcomes.
In thinking about which EUS-FNA needle is best for transmural puncture in EUS-BD, the vast majority of published cases report using a 19 G needle. The consortium group in 2011 stated that this is the optimal needle because of its appropriate stiffness, which allows for an effective transfer of force, and also its excellent tip visibility on both EUS and fluoroscopy imaging.10 Although the stiffness of this needle may be an obstacle in certain situations when needle positioning or maneuvering is crucial to obtain the proper entry angle. In a recent large multicenter international study with interventional endoscopy experts reporting their experience in 240 EUS-BD cases, over 99% of the cases were performed with 19 G needles.12 That is not to say, however, that this is the only successful approach as a recent prospective single center study reported 100% success rate of biliary puncture using a 22 G needle in combination with a diathermic needle-knife.13 As of now, there are no randomized controlled trials (RCTs) comparing the outcomes of various needle calibers in EUS-BD. Newer devices are being developed, such as diathermic needle knives with removable inner needles. These devices provide pure cutting current to allow for easy tissue penetration, and exchange of the inner needle for a guidewire, however the concern is for an increased risk of thermal injury.10
In most cases of EUS-BD, except for rendezvous drainage, transmural tract dilation will be required. The most common tools for this are bougies and dilating balloons. Bougies require manual advancement through the fistula tract by the endoscopist, which gives the operator some tactile sense of the degree of tissue resistance. This may be helpful as it provides the endoscopist with more information and help prevent excessive tissue injury. However, because a portion of the force exerted by the bougie acts tangentially to the plane of the tissue, this may lead to increased tissue separation and complications.10 Generally a 6 to 7 Fr bougie is optimal for dilating a transmural fistula to the necessary size for further wire manipulation and stent placement. Dilating balloons on the other hand transmit all their force in a radial fashion in the same plane of the tissue being dilated, thus minimizing the shearing force. The compressibility of balloons to about a 5 Fr delivery system makes them convenient for passing through the fistula after needle puncture. However, because balloons are inflated mechanically, the feedback of tissue resistance is lost to the endoscopist and the results of the dilation are only seen after the balloon is deflated, which may increase the risk for complications such as bleeding, perforation and leakage.10 These 4- to 6-mm dilating balloons are usually adequate for tract dilation.
The options for stent placement include plastic (straight or pigtail) and metal (covered or partially covered) stents. Uncovered metal stents should not be used for formation of a transmural fistula to avoid the risk of leakage and peritonitis. Uncovered stents may, however, be appropriate for temporary placement in mature fistula tracts. Although no comparative studies have been published in EUS-BD, CSEMS are thought to provide longer patency rates based on previously published RCTs.14,15 Also retrospective data suggests that CSEMS may have lower rates of postoperative cholangitis in EUS-BD compared to plastic stents, although this needs to be confirmed in prospective studies.12 The concern with CSEMS is the potential to migrate, shorten or occlude secondary ducts (intrahepatic radicals, cystic duct, etc).10 Most case series, however, have reported low migration rates with CSEMS. A large retrospective series of EUS-BD including 248 patients with either CSEMS or plastic stent placement resulted in an overall migration rate of 3.2%.16 Anecdotal tactics to minimize the risk of migration include placing a double-pigtail plastic stent through the CSEMs.
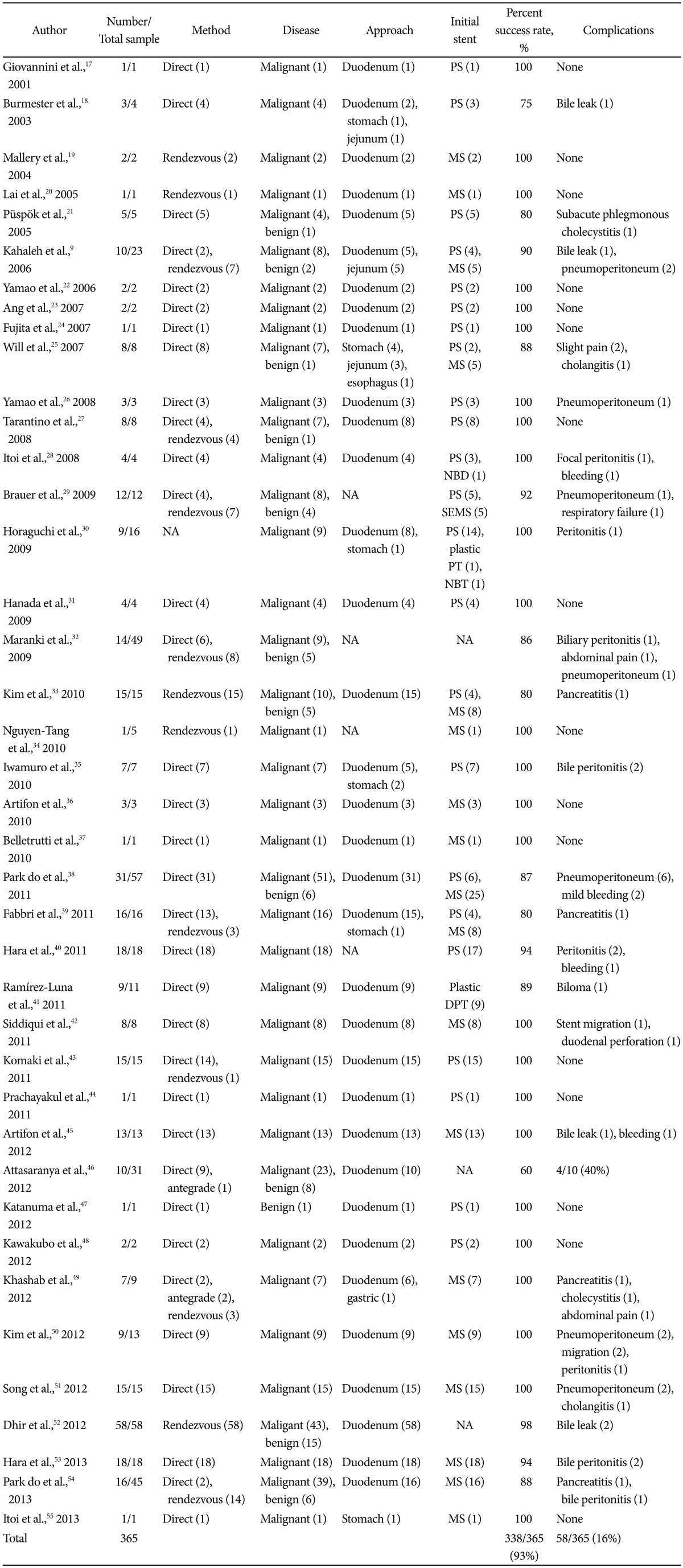
Presently, the majority of the reported outcomes on EUS-BD are in the form of small retrospective case series with few prospective studies and RCTs. However the data, as seen in Table 1, is promising as the cumulative success rate for extrahepatic EUS-BD is approximately 93% over the past 12 years.9,17-55
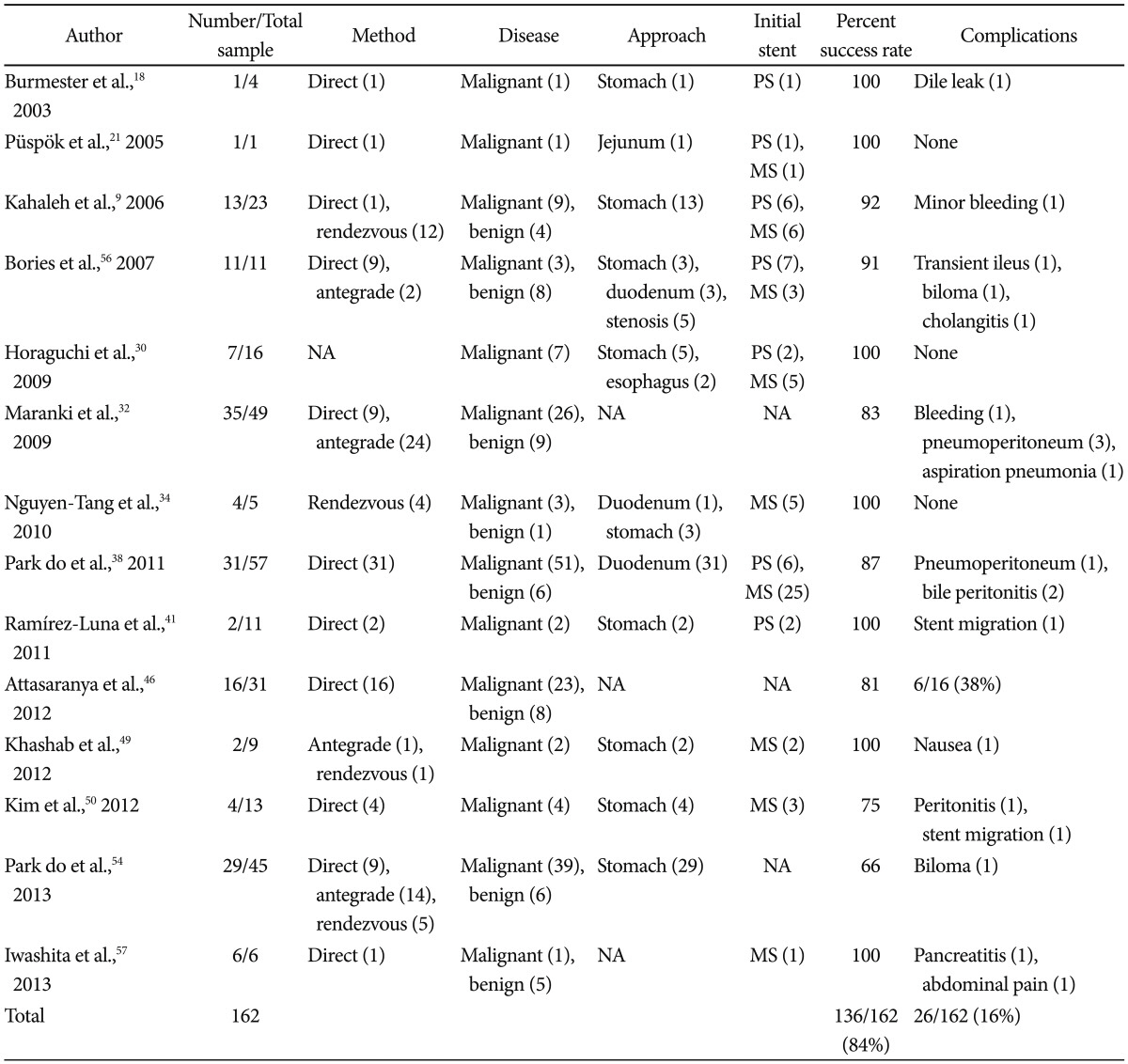
Table 2 summarizes the published literature on intrahepatic EUS-BD, which shows a cumulative success rate of 84%.9,18,21,30,32,34,38,41,46,49-51,56,57
There are no prospective studies comparing outcomes between the various possible drainage approaches in EUS-BD. Two large multicenter retrospective studies did not show any difference in the success rates between intrahepatic or extrahepatic drainage routes.12,16 One of the reviews could not predict success or complications based on gender, stricture type, access point (intrahepatic or extrahepatic), stent route placement, thus implying that treatment should be individualized for each case.16 Limited study has been performed in evaluating outcomes of EUS-BD compared to other salvage biliary techniques such as precut sphincterotomy, percutaneous BD, and surgery. One retrospective comparative study revealed that EUS-BD had a significantly higher technical success rate compared to precut sphincterotomy in cases of failed ERCP.52 Also, one small, RCT comparing clinical outcomes in patients having received EUS-BD versus percutaneous transhepatic BD showed no significant difference in success rates, although patient satisfaction and pain scores were not reported.45 Further prospective multicenter trials are needed to decipher where EUS-BD should lie in the treatment algorithm for these patients.
The performance of EUS-BD is limited by need for endoscopic expertise and training along with the appropriate interventional radiology and surgical backup to manage potential procedure failure and complications. Also because of the novelty of the technique, dedicated devices have not yet been developed to improve procedure outcomes. There are multiple intricate aspects of EUS-BD such as transmural needle puncture, wire manipulation, tract dilation, stent placement, and endoscope exchanges that may lead to complications if not performed by an experienced, expert endoscopist. The most common complications associated with EUS-BD include: 1) infection (peritonitis, cholangitis, cholecystitis), 2) pancreatitis, 3) pneumoperitoneum, 4) bile leak/biloma, 5) bleeding, 6) abdominal pain, and 7) stent migration. Rare serious adverse events include sepsis and perforation. Based on the currently available literature the overall complication rate for EUS-BD is around 16%, most of which fortunately can be managed conservatively (Tables 1, 2). The clinical significance of some events such as pneumoperitoneum have yet to be determined, and may eventually become expected consequences like when performing percutaneous gastrostomy. Very few comparative studies looking at EUS-BD versus alternative drainage procedures exist. Two comparative studies have shown no difference in complication rates when comparing EUS-BD to precut sphincterotomy or percutaneous transhepatic BD.45,52 Larger prospective, preferably randomized multicenter trials are needed to substantiate these complication rates.
EUS-BD is a novel procedure that has been quickly gaining acceptance in recent years as an alternative option for BD in patients having previously failed ERCP. Multiple retrospective and some prospective studies have shown it to be an effective and safe procedure in the hands of an expert pancreaticobiliary endoscopist. Based on the currently reported literature, the cumulative success rate, regardless of EUS-BD approach, is about 90% with an overall complication rate of about 16%. Although comparative studies are currently lacking, EUS-BD theoretically confers logistical, economic, and anatomic/physiologic benefits compared to more invasive options like percutaneous and surgical drainage. Indications and methods for EUS-BD have yet to be standardized therefore the approach should be individualized for each patient based on the endoscopist's assessment. CSEMS may result in longer patency rates and lower rates of cholangitis based on existing data. Further prospective, multicenter, controlled studies are needed to further delineate appropriate indications, predictors of success and complications, optimal approach, and clinical outcomes compared to other drainage procedures.
References
1. Baron TH, Petersen BT, Mergener K, et al. Quality indicators for endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 2006; 63(4 Suppl):S29–S34. PMID: 16564909.

2. Puspok A, Lomoschitz F, Dejaco C, Hejna M, Sautner T, Gangl A. Endoscopic ultrasound guided therapy of benign and malignant biliary obstruction: a case series. Am J Gastroenterol. 2005; 100:1743–1747. PMID: 16086710.

3. Horaguchi J, Fujita N, Noda Y, et al. Endosonography-guided biliary drainage in cases with difficult transpapillary endoscopic biliary drainage. Dig Endosc. 2009; 21:239–244. PMID: 19961522.

4. Ferrucci JT Jr, Mueller PR, Harbin WP. Percutaneous transhepatic biliary drainage: technique, results, and applications. Radiology. 1980; 135:1–13. PMID: 7360943.

5. Harbin WP, Mueller PR, Ferrucci JT Jr. Transhepatic cholangiography: complicatons and use patterns of the fine-needle technique: a multi-institutional survey. Radiology. 1980; 135:15–22. PMID: 6987704.

6. Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994; 344:1655–1660. PMID: 7996958.
7. Wiersema MJ, Sandusky D, Carr R, Wiersema LM, Erdel WC, Frederick PK. Endosonography-guided cholangiopancreatography. Gastrointest Endosc. 1996; 43(2 Pt 1):102–106. PMID: 8635700.

8. Giovannini M, Dotti M, Bories E, et al. Hepaticogastrostomy by echoendoscopy as a palliative treatment in a patient with metastatic biliary obstruction. Endoscopy. 2003; 35:1076–1078. PMID: 14648424.

9. Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006; 64:52–59. PMID: 16813803.

10. Kahaleh M, Artifon EL, Perez-Miranda M, et al. Endoscopic ultrasonography guided biliary drainage: summary of consortium meeting, May 7th, 2011, Chicago. World J Gastroenterol. 2013; 19:1372–1379. PMID: 23538784.

11. Sarkaria S, Lee HS, Gaidhane M, Kahaleh M. Advances in endoscopic ultrasound-guided biliary drainage: a comprehensive review. Gut Liver. 2013; 7:129–136. PMID: 23560147.

12. Gupta K, Perez-Miranda M, Kahaleh M, et al. Endoscopic ultrasound-assisted bile duct access and drainage: multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J Clin Gastroenterol. Epub 2013 Apr 29. DOI: 10.1097/MCG.0b013e31828c6822.
13. Hara K, Yamao K, Hijioka S, et al. Prospective clinical study of endoscopic ultrasound-guided choledochoduodenostomy with direct metallic stent placement using a forward-viewing echoendoscope. Endoscopy. 2013; 45:392–396. PMID: 23338620.

14. Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomized trial of self expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992; 340:1488–1492. PMID: 1281903.
15. Kaassis M, Boyer J, Dumas R, et al. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003; 57:178–182. PMID: 12556780.

16. Kahaleh M, Perez-Miranda M, Artifon EL, et al. 140 Endoscopic ultrasound (EUS) guided biliary drainage: what have we learned? Gastrointest Endosc. 2013; 77(5 Suppl):AB127–AB128.

17. Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001; 33:898–900. PMID: 11571690.

18. Burmester E, Niehaus J, Leineweber T, Huetteroth T. EUS-cholangiodrainage of the bile duct: report of 4 cases. Gastrointest Endosc. 2003; 57:246–251. PMID: 12556796.

19. Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: report of 6 cases. Gastrointest Endosc. 2004; 59:100–107. PMID: 14722561.

20. Lai R, Freeman ML. Endoscopic ultrasound-guided bile duct access for rendezvous ERCP drainage in the setting of intradiverticular papilla. Endoscopy. 2005; 37:487–489. PMID: 15844030.

21. Püspök A, Lomoschitz F, Dejaco C, Hejna M, Sautner T, Gangl A. Endoscopic ultrasound guided therapy of benign and malignant biliary obstruction: a case series. Am J Gastroenterol. 2005; 100:1743–1747. PMID: 16086710.
22. Yamao K, Sawaki A, Takahashi K, Imaoka H, Ashida R, Mizuno N. EUS-guided choledochoduodenostomy for palliative biliary drainage in case of papillary obstruction: report of 2 cases. Gastrointest Endosc. 2006; 64:663–667. PMID: 16996372.

23. Ang TL, Teo EK, Fock KM. EUS-guided transduodenal biliary drainage in unresectable pancreatic cancer with obstructive jaundice. JOP. 2007; 8:438–443. PMID: 17625296.
24. Fujita N, Noda Y, Kobayashi G, et al. Histological changes at an endosonography-guided biliary drainage site: a case report. World J Gastroenterol. 2007; 13:5512–5515. PMID: 17907298.

25. Will U, Thieme A, Fueldner F, Gerlach R, Wanzar I, Meyer F. Treatment of biliary obstruction in selected patients by endoscopic ultrasonography (EUS)-guided transluminal biliary drainage. Endoscopy. 2007; 39:292–295. PMID: 17357950.

26. Yamao K, Bhatia V, Mizuno N, et al. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: results of long-term follow-up. Endoscopy. 2008; 40:340–342. PMID: 18389451.

27. Tarantino I, Barresi L, Repici A, Traina M. EUS-guided biliary drainage: a case series. Endoscopy. 2008; 40:336–339. PMID: 18264890.

28. Itoi T, Itokawa F, Sofuni A, et al. Endoscopic ultrasound-guided choledochoduodenostomy in patients with failed endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2008; 14:6078–6082. PMID: 18932289.

29. Brauer BC, Chen YK, Fukami N, Shah RJ. Single-operator EUS-guided cholangiopancreatography for difficult pancreaticobiliary access (with video). Gastrointest Endosc. 2009; 70:471–479. PMID: 19560768.

30. Horaguchi J, Fujita N, Noda Y, et al. Endosonography-guided biliary drainage in cases with difficult transpapillary endoscopic biliary drainage. Dig Endosc. 2009; 21:239–244. PMID: 19961522.

31. Hanada K, Iiboshi T, Ishii Y. Endoscopic ultrasound-guided choledochoduodenostomy for palliative biliary drainage in cases with inoperable pancreas head carcinoma. Dig Endosc. 2009; 21(Suppl 1):S75–S78. PMID: 19691742.

32. Maranki J, Hernandez AJ, Arslan B, et al. Interventional endoscopic ultrasound-guided cholangiography: long-term experience of an emerging alternative to percutaneous transhepatic cholangiography. Endoscopy. 2009; 41:532–538. PMID: 19533558.

33. Kim YS, Gupta K, Mallery S, Li R, Kinney T, Freeman ML. Endoscopic ultrasound rendezvous for bile duct access using a transduodenal approach: cumulative experience at a single center. A case series. Endoscopy. 2010; 42:496–502. PMID: 20419625.

34. Nguyen-Tang T, Binmoeller KF, Sanchez-Yague A, Shah JN. Endoscopic ultrasound (EUS)-guided transhepatic anterograde self-expandable metal stent (SEMS) placement across malignant biliary obstruction. Endoscopy. 2010; 42:232–236. PMID: 20119894.

35. Iwamuro M, Kawamoto H, Harada R, et al. Combined duodenal stent placement and endoscopic ultrasonography-guided biliary drainage for malignant duodenal obstruction with biliary stricture. Dig Endosc. 2010; 22:236–240. PMID: 20642617.

36. Artifon EL, Takada J, Okawa L, Moura EG, Sakai P. EUS-guided choledochoduodenostomy for biliary drainage in unresectable pancreatic cancer: a case series. JOP. 2010; 11:597–600. PMID: 21068493.
37. Belletrutti PJ, Gerdes H, Schattner MA. Successful endoscopic ultrasound-guided transduodenal biliary drainage through a pre-existing duodenal stent. JOP. 2010; 11:234–236. PMID: 20442518.
38. Park do H, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011; 74:1276–1284. PMID: 21963067.
39. Fabbri C, Luigiano C, Fuccio L, et al. EUS-guided biliary drainage with placement of a new partially covered biliary stent for palliation of malignant biliary obstruction: a case series. Endoscopy. 2011; 43:438–441. PMID: 21271507.

40. Hara K, Yamao K, Niwa Y, et al. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol. 2011; 106:1239–1245. PMID: 21448148.

41. Ramírez-Luna MA, Téllez-Ávila FI, Giovannini M, Valdovinos-Andraca F, Guerrero-Hernández I, Herrera-Esquivel J. Endoscopic ultrasound-guided biliodigestive drainage is a good alternative in patients with unresectable cancer. Endoscopy. 2011; 43:826–830. PMID: 21833899.

42. Siddiqui AA, Sreenarasimhaiah J, Lara LF, Harford W, Lee C, Eloubeidi MA. Endoscopic ultrasound-guided transduodenal placement of a fully covered metal stent for palliative biliary drainage in patients with malignant biliary obstruction. Surg Endosc. 2011; 25:549–555. PMID: 20632191.

43. Komaki T, Kitano M, Sakamoto H, Kudo M. Endoscopic ultrasonographyguided biliary drainage: evaluation of a choledochoduodenostomy technique. Pancreatology. 2011; 11(Suppl 2):47–51. PMID: 21464587.

44. Prachayakul V, Aswakul P, Kachintorn U. EUS-guided choledochoduodenostomy for biliary drainage using tapered-tip plastic stent with multiple fangs. Endoscopy. 2011; 43(Suppl 2 UCTN):E109–E110. PMID: 21424999.

45. Artifon EL, Aparicio D, Paione JB, et al. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012; 46:768–774. PMID: 22810111.
46. Attasaranya S, Netinasunton N, Jongboonyanuparp T, et al. The spectrum of endoscopic ultrasound intervention in biliary diseases: a single center's experience in 31 cases. Gastroenterol Res Pract. 2012; 2012:680753. PMID: 22654900.

47. Katanuma A, Maguchi H, Osanai M, Takahashi K. Endoscopic ultrasound-guided biliary drainage performed for refractory bile duct stenosis due to chronic pancreatitis: a case report. Dig Endosc. 2012; 24(Suppl 1):34–37. PMID: 22533749.

48. Kawakubo K, Isayama H, Nakai Y, et al. Simultaneous duodenal metal stent placement and EUS-guided choledochoduodenostomy for unresectable pancreatic cancer. Gut Liver. 2012; 6:399–402. PMID: 22844572.

49. Khashab MA, Fujii LL, Baron TH, et al. EUS-guided biliary drainage for patients with malignant biliary obstruction with an indwelling duodenal stent (with videos). Gastrointest Endosc. 2012; 76:209–213. PMID: 22726485.

50. Kim TH, Kim SH, Oh HJ, Sohn YW, Lee SO. Endoscopic ultrasound-guided biliary drainage with placement of a fully covered metal stent for malignant biliary obstruction. World J Gastroenterol. 2012; 18:2526–2532. PMID: 22654450.

51. Song TJ, Hyun YS, Lee SS, et al. Endoscopic ultrasound-guided choledochoduodenostomies with fully covered self-expandable metallic stents. World J Gastroenterol. 2012; 18:4435–4440. PMID: 22969210.

52. Dhir V, Bhandari S, Bapat M, Maydeo A. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos). Gastrointest Endosc. 2012; 75:354–359. PMID: 22248603.

53. Hara K, Yamao K, Hijioka S, et al. Prospective clinical study of endoscopic ultrasound-guided choledochoduodenostomy with direct metallic stent placement using a forward-viewing echoendoscope. Endoscopy. 2013; 45:392–396. PMID: 23338620.

54. Park do H, Jeong SU, Lee BU, et al. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video). Gastrointest Endosc. 2013; 78:91–101. PMID: 23523301.
55. Itoi T, Itokawa F, Tsuchiya T, Tsuji S, Tonozuka R. Endoscopic ultrasound-guided choledochoantrostomy as an alternative extrahepatic bile duct drainage method in pancreatic cancer with duodenal invasion. Dig Endosc. 2013; 25(Suppl 2):142–145. PMID: 23617666.

56. Bories E, Pesenti C, Caillol F, Lopes C, Giovannini M. Transgastric endoscopic ultrasonography-guided biliary drainage: results of a pilot study. Endoscopy. 2007; 39:287–291. PMID: 17357952.
57. Iwashita T, Yasuda I, Doi S, et al. Endoscopic ultrasound-guided antegrade treatments for biliary disorders in patients with surgically altered anatomy. Dig Dis Sci. 2013; 58:2417–2422. PMID: 23535877.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download