Abstract
Gastrointestinal endoscopy is gaining popularity for diagnostic and therapeutic purposes. However, concerns over endoscope-related nosocomial infections are increasing, together with interest by the general public in safe and efficient endoscopy. For this reason, reprocessing the gastrointestinal endoscope is an important step for effective performance of endoscopy. Disinfectants are essential to the endoscope reprocessing procedure. Before selecting an appropriate disinfectant, their characteristics, limitations and means of use must be fully understood. Herein, we review the characteristics of several currently available disinfectants, including their uses, potency, advantages, and disadvantages. Most disinfectants can be used to reprocess gastrointestinal endoscopes if the manufacturer's guidelines are followed. The selection and use of a suitable disinfectant depends on the individual circumstances of each endoscopy suite.
Go to : 
Gastrointestinal (GI) endoscopy is an essential component in the diagnosis and treatment of GI diseases. Being reusable devices, GI endoscopes inevitably encounter pathogens that may contaminate subsequent patients, especially those who are immunosuppressed due to age or concurrent disease. Although the estimated rate of transmission of infection via GI endoscopy is extremely low at 1 in 1.8 million procedures,1-3 many flaws and deficiencies in endoscope reprocessing can result in microbial transmission.4 Therefore, disinfection is an essential component of the reprocessing of endoscopes between patients.
Since GI endoscopes contact human mucosa during use, they are classified as a semicritical apparatus requiring high level disinfection.5 Endoscopes do not require full sterilization, unlike packaged medical products (e.g., syringes and disposable medical apparatuses); in fact, it is fundamentally impossible to sterilize an endoscope via autoclave or ethylene oxide sterilization, considering its structure, the materials composing the equipment, and because durability may be compromised by such processes.6
Typically, reprocessing a GI endoscope consists of four steps: cleaning, disinfection, rinsing, and drying/storing. In this process, disinfectants are required to remove bacteria, bacterial spores, viruses, fungi, and acid-fast bacilli. Either mechanically or manually, potential pathogens should be eliminated during disinfection between procedures. A number of disinfectants are available, six of which have been approved by the U.S. Food and Drug Administration: glutaraldehyde (GA), orthophthalaldehyde (OPA), peracetic acid (PAA), hydrogen peroxide (HPO), electrolyzed acid water (EAW), and PAA/HPO blend.7 In other countries, some other disinfectants are approved for use, including chlorine dioxide (ClO2), peroxygen compounds, quaternary ammonium, and ozonated water.
Recently, the Korean public's concern over endoscope-mediated nosocomial infection has escalated after several reports of imperfect cleaning of endoscopes between patients, leading to increased social interest in safe and efficient GI endoscopy. As proper endoscope reprocessing became imperative, thorough education and instruction became important for medical professionals working in the endoscopy suite. In 2012, the Korean Society of Gastrointestinal Endoscopy revised its disinfection guidelines for reprocessing endoscopes and published the new protocol, which included the appropriate disinfectants.8 Herein, we will briefly discuss several disinfectants commonly used in Korea in terms of their uses, potency, advantages, and disadvantages.
Go to : 
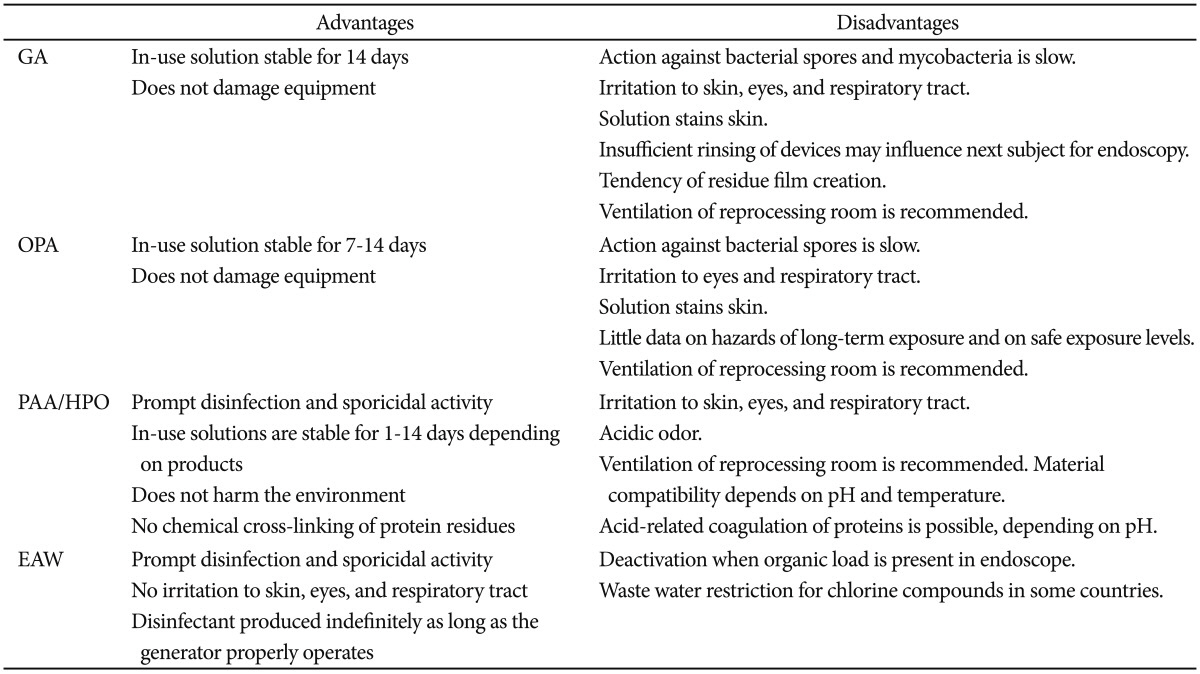
GA is the most commonly used disinfectant worldwide.9 GA performs best via activation when diluted as a 2% (range, 2.4 to 3.5) solution of pH 7.5 to 8.5 by addition of a buffer solution (e.g., sodium bicarbonate, sodium phosphate), since GA is an unstable acid in its crude liquid form. High level disinfection is feasible with 20 minutes of exposure at 20℃ in a 2% GA solution without surfactant.10 The advantages of GA are that it is an inexpensive, efficient disinfectant with minimal damage to endoscopic apparatuses.11 Currently available solution is stable for a long time, and it can be used repeatedly for as long as 14 days. In Korea, Cidex (Johnson and Johnson Medical, Seoul, Korea) and Widex (Dongindang Pharmaceutical Co., Ltd., Siheung, Korea) are representative products on the market.
The main limitation of GA compared with other disinfectants is its relatively lengthy immersion time (around 20 minutes; 45 minutes for Mycobacterium and 6 to 10 hours for spores) at 25℃.12 Protein coagulation in the endoscope is another major disadvantage, as GA may accumulate or clog the working channel of the endoscope in the presence of blood or other debris, consequently reducing the disinfection efficiency.
GA may cause serious irritation13,14 to the eyes or respiratory mucosa, and can cause allergic reactions of the skin, nose, ear, and pharynx; bronchial asthma and rhinitis have also been reported following GA exposure. Improper rinsing of GA after disinfection can be harmful to the next patient, incurring abdominal pain and other symptoms of gastroenteritis.15-17 In terms of the limit in the number of use, dilution of the GA solution (under 2%) for repeated use may lead to decreased effectiveness.
OPA is also an aldehyde disinfectant, containing an aromatic aldehyde. This chemical overcomes several shortcomings of GA, and therefore shows superior reactivity.20,21 OPA does not require activation by dilution with buffer solution, but can be used directly from the container. Similar to GA, organic debris left in the endoscope can form a residue, but this is easily washed away upon irrigation. Immersion time for disinfection is shorter than GA at only 5 minutes. As it has less vaporizing activity and less toxicity, OPA is safer for reprocessing personnel.
OPA is generally more costly than GA, and may permanently stain skin or clothing black. Although OPA can be used for as long as 14 consecutive days, an exclusive test strip is needed for verification. OPA can also be an irritant for workers involved in endoscope treatment.22
PAA is a less toxic, more powerful, and more rapidly disinfecting agent compared to GA and OPA. Additional oxygen is added to one acetic acid molecule (CH3COOH) to form PAA, which produces nascent oxygen when mixed with water, conferring the disinfection properties. An acidic environment is preferred for PAA stability, but this may corrode the metallic components of endoscopes. Thus, a buffer and metal protecting solution are added when PAA is used as a disinfectant.
PAA acts through various mechanisms of decontamination with a lower possibility of resistance generation, as compared with GA. It denatures proteins, inhibits cell transport, inactivates essential metabolic enzymes, degrades cell membranes, and denatures nucleic acids.23 PAA also leaves no protein coagulation or precipitation on endoscopes. The shorter time required for disinfection (5 minutes for bacteria and 10 minutes for tubercle bacilli) is another advantage of PAA.
However, HPO acts by producing free hydroxyl radicals, which denature cell walls and bacterial enzymes.24 The working concentration varies from 3% to 25%. PAA and HPO are good decontaminants when used alone, but a synergistic effect can be achieved when they are mixed. PeraSafe (Antec International Ltd., Sudbury, UK), Scotelin (KR&D, Busan, Korea), and Acecide (Saraya Co., Ltd., Osaka, Japan) are the representative blended products sold in Korea.
The most significant limitation of PAA is its price. In addition, the acidity of PAA may corrode endoscopes (especially metallic parts). As it is unstable after activation, some PAA products cannot be used after 24 hours. Low water temperature and high pH may lower the efficacy of some PAA products; 35℃ of water is needed for optimal disinfection. As manual disinfection is impossible, an automated washer is required.
Electrolysis of weak (0.5%) NaCl solution with tap water produces hydrogen ions (H+), hypochlorous acid (HClO), and chlorine (Cl2). The latter two elements (Cl2 and HClO) confer the disinfectant properties, denaturing nucleic acids, and inactivating enzymes. The disinfectant converts into water when exposed to light or ambient air, leaving behind no harmful side products, giving EAW a very low irritability and toxicity. Immersion time is about 7 minutes, shorter than other disinfectants. The maintenance costs are significantly lower due to the use of tap water and NaCl. A low probability of resistance is an added benefit of EAW.
EAWs are subdivided into strongly acidic, weakly acidic, weakly alkaline, and strongly alkaline solutions.25 Strong acidity leads to increased disinfection potency, but this accompanies increased metal corrosion and toxic Cl2 gas production. Medilox (Soosan E&C Co., Ltd., Seongnam, Korea) is a disinfectant with a pH of 5.0 to 7.0, while Cleantop WM-1 (Kaigen, Osaka, Japan) produces a disinfectant liquid with a pH of 2.5±0.2.26 Weakly acidic EAW with a pH value of 5.0 to 6.0 has a higher HClO concentration, making it a more suitable disinfectant.27,28
The shelf life is relatively short at about 24 hours. EAW is vulnerable to ambient air exposure with the possibility of toxic Cl2 gas production, is weakly effective against tubercle bacilli,27 and is likely to desiccate endoscopes on prolonged immersion. A small quantity of organic compounds left in the endoscope before the disinfection process will decrease the disinfection efficacy.4
Go to : 
Free Cl2 ions are generated from this disinfectant, enabling rapid, and wide-ranging disinfection. However, the strong Cl2 dioxide fumes from this substance irritate the human respiratory tract, and can erode endoscopic instruments.29 Although currently used as a tap water disinfectant in Korea, CDO is not employed in endoscope reprocessing.
With its strong acidity, ozonated water is known to have a quick disinfecting action. Tap water is directly transformed into a decontaminant, which results in a low maintenance requirement. However, the disinfection effectiveness of ozonated water has yet to be validated.27
Because of its low disinfectant potency (not tuberculocidal or sporicidal, poor activity against hydrophilic viruses),24 this chemical is not recommended for endoscope reprocessing.
Go to : 
Several factors should be considered when choosing a disinfectant, such as the time required for disinfection, convenience of usage, corrodibility of apparatuses, cost, toxicity to personnel, and requirement of a cleanser machine. The endoscopic suites of each institution differ in terms of capacity, types of endoscopy performed, disinfection facilities and the presence of exclusive reprocessing personnel; these factors are also important in the selection of a disinfectant. In general, most disinfectants are sufficient for reprocessing GI endoscopes if the manufacturer's instructions on their use and maintenance are followed.
Go to : 
References
1. Nelson DB. Recent advances in epidemiology and prevention of gastrointestinal endoscopy related infections. Curr Opin Infect Dis. 2005; 18:326–330. PMID: 15985829.

2. Spach DH, Silverstein FE, Stamm WE. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Med. 1993; 118:117–128. PMID: 8416308.

3. Nelson DB, Barkun AN, Block KP, et al. Technology status evaluation report Transmission of infection by gastrointestinal endoscopy. May 2001. Gastrointest Endosc. 2001; 54:824–828. PMID: 11726877.
4. Beilenhoff U, Neumann CS, Rey JF, et al. ESGE-ESGENA Guideline: cleaning and disinfection in gastrointestinal endoscopy. Endoscopy. 2008; 40:939–957. PMID: 19009486.

5. Favero MS, Bond WW. Disinfection of medical and surgeical materials. In : Block SS, editor. Disinfection, Sterilization, and Preservation. 5th ed. Philadelphia: Lippincott Williams & Wilkins;2001. p. 881–917.
6. Kim HJ. KFDA-cleared disinfectants in gastrointestinal endoscopy. Korean J Gastrointest Endosc. 2010; 40:50–52.
7. U.S. Food and Drug Administration. FDA-cleared sterilants and high level disinfectants with general claims for processing reusable medical and dental devices: March 2009 [Internet]. Silver Spring: U.S. Food and Drug Administration;2009. updated 2009 Apr 26. cited 2012 Feb 2. Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/ReprocessingofSingle-UseDevices/ucm133514.htm.
8. The Korean Society of Gastrointestinal Endoscopy Disinfection Management Committee. Guidebook for Cleaning and Disinfecting Gastrointestinal Endoscopes. 1st ed. Seoul: Medbook;2012. p. 93.
9. Society of Gastroenterology Nurses and Associates. SGNA guidelines for nursing care of the patient receiving sedation and analgesia in the gastrointestinal endoscopy setting. Gastroenterol Nurs. 2000; 23:125–129. PMID: 11235444.
10. Walter V. Reprocessing of flexible gastrointestinal endoscopes: an American Society for Gastrointestinal Endoscopy white paper. Gastroenterol Nurs. 1996; 19:109–112. PMID: 8716956.
11. Rutala WA. Association for Professionals in Infection Control and Epidemiology, Inc. APIC guideline for selection and use of disinfectants. 1994, 1995, and 1996 APIC Guidelines Committee. Am J Infect Control. 1996; 24:313–342. PMID: 8870916.
12. Griffiths PA, Babb JR, Fraise AP. Mycobactericidal activity of selected disinfectants using a quantitative suspension test. J Hosp Infect. 1999; 41:111–121. PMID: 10063473.

13. Axon AT, Banks J, Cockel R, Deverill CE, Newmann C. Disinfection in upper-digestive-tract endoscopy in Britain. Lancet. 1981; 1:1093–1094. PMID: 6112457.

14. Cowan RE, Manning AP, Ayliffe GA, et al. British Society of Gastroenterology Endoscopy Committee. Aldehyde disinfectants and health in endoscopy units. Gut. 1993; 34:1641–1645. PMID: 8244157.

15. Hanson JM, Plusa SM, Bennett MK, Browell DA, Cunliffe WJ. Glutaraldehyde as a possible cause of diarrhoea after sigmoidoscopy. Br J Surg. 1998; 85:1385–1387. PMID: 9782020.

16. West AB, Kuan SF, Bennick M, Lagarde S. Glutaraldehyde colitis following endoscopy: clinical and pathological features and investigation of an outbreak. Gastroenterology. 1995; 108:1250–1255. PMID: 7698592.

17. Jonas G, Mahoney A, Murray J, Gertler S. Chemical colitis due to endoscope cleaning solutions: a mimic of pseudomembranous colitis. Gastroenterology. 1988; 95:1403–1408. PMID: 3169504.

18. Jordan SL. The correct use of glutaraldehyde in the healthcare environment. Gastroenterol Nurs. 1995; 18:143–145. PMID: 7654811.

19. Burkart J. NIOSH Health Hazard Evaluation Report. Washington, DC: U.S. Dept. of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health;1991.
20. Gregory AW, Schaalje GB, Smart JD, Robison RA. The mycobactericidal efficacy of ortho-phthalaldehyde and the comparative resistances of Mycobacterium bovis, Mycobacterium terrae, and Mycobacterium chelonae. Infect Control Hosp Epidemiol. 1999; 20:324–330. PMID: 10349948.

21. Alfa MJ, Sitter DL. In-hospital evaluation of orthophthalaldehyde as a high level disinfectant for flexible endoscopes. J Hosp Infect. 1994; 26:15–26. PMID: 7910179.

22. Rideout K, Teschke K, Dimich-Ward H, Kennedy SM. Considering risks to healthcare workers from glutaraldehyde alternatives in high-level disinfection. J Hosp Infect. 2005; 59:4–11. PMID: 15571847.

23. Kim KJ. Pros and cons of various endoscopic disinfectants. Korean J Gastrointest Endosc. 2009; 39(Suppl):97S–100S.
25. Kim H. Characteristics and types of disinfectants. Korean J Gastrointest Endosc. 2010; 41:198–201.
26. Lee JH, Rhee PL, Kim JH, et al. Efficacy of electrolyzed acid water in reprocessing patient-used flexible upper endoscopes: comparison with 2% alkaline glutaraldehyde. J Gastroenterol Hepatol. 2004; 19:897–903. PMID: 15242493.

27. Urata M, Isomoto H, Murase K, et al. Comparison of the microbicidal activities of superoxidized and ozonated water in the disinfection of endoscopes. J Int Med Res. 2003; 31:299–306. PMID: 12964505.

28. Rossi-Fedele G, Guastalli AR, Doğramacı EJ, Steier L, De Figueiredo JA. Influence of pH changes on chlorine-containing endodontic irrigating solutions. Int Endod J. 2011; 44:792–799. PMID: 21658076.

29. BSG Endoscopy Committee Working Party. Cleaning and disinfection of equipment for gastrointestinal endoscopy. Report of a Working Party of the British Society of Gastroenterology Endoscopy Committee. Gut. 1998; 42:585–593. PMID: 9616326.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download