Abstract
This paper reviews the use of photodynamic therapy (PDT) in patients with Barrett's esophagus and esophageal carcinoma. We describe the history of PDT, mechanics, photosensitizers for PDT in patients with esophageal disease. Finally, we discuss its utility and limitations in this setting.
Photodynamic therapy (PDT) uses photosensitizer drugs that are activated by light energy and are then able to interact with oxygen or other vascular and cellular components to stimulate a photodynamic reaction often producing damage or necrosis to the target tissue. This treatment effect occurs in all sensitized cells (such as normal esophageal mucosa, as well as dysplastic or neoplastic mucosa) that are exposed to a light source, in proportion of the mucosal concentration of the photosensitizer drug (often determined by mucosal blood flow and oxygenation status) and dose of light energy. PDT has been used for decades to treat diseased esophageal mucosa in the setting of squamous dysplasia and carcinoma, as well as Barrett's mucosa with dysplasia or neoplasia.1
Many agents have been used as photosensitizers in esophageal disease. The initial studies were focused on patients with esophageal cancer and the use of porfimer sodium (Ps) PDT.2,3 Such research led to approval of PDT in the United States, Europe, and Japan. Later, Overholt et al.4,5 published a landmark study on the use of Ps PDT and patients with Barrett's esophagus (BE) with high grade dysplasia (HGD). This trial was a prospective, randomized, controlled, multicenter study and results of the study were published in three major research articles.4,5 The trial showed a lower progression rate to invasive esophageal carcinoma among patients who were treated with PDT. Since then, several studies demonstrated the clinical usefulness of Ps PDT on BE patient with dysplasia and neoplasia which has resulted in the development of other photosensitizers (Table 1).6-15
The initial description of PDT using porphyrin-based agents, hematoporphyrin derivative (HpD) and dihematoporphyrin ether (DHE) in combination with red light, became the predominant forms of PDT.16 After further purification of the photosensetizers, several agents were released: Ps (Photofrin; Pinnacle Biologics Inc., Bannockburn, IL, USA),17 DHE (available in Europe under the trade name Photosan [Seehof Laboratories, Wesselburen, Germany], a second-generation form of HpD available in China under the trade name Hemporfin [hematoporphyrin monomethyl ether; Funda-Zhangjiang Bio-Pharmaceutical, Shanghai, China]).18 Ps has recently undergone pharmacokinetic studies after repeated administration (demonstrating prolonged half-life and delayed excretion after repeated infusion).19 A recent review from Nara, Japan, has also described advances in the research and development of 5-aminolevulinic acid (ALA) for PDT, as well as the use of nonporphyrinoid compounds.20
When reviewing the literature about PDT, one needs to pay attention to three major points of interest. First of those points is the disease status of the study population. The results of a study on patient with BE and low grade dysplasia (LGD) should be interpreted differently from that of patients with HGD or early carcinoma (EC) and squamous dysplasia or carcinoma. Second, one requires a basic understanding of the physics and mechanics of the PDT technique. This includes how different photosensitizers are used, the various light sources, and the routes of administration. Equivalent doses of various agents may result in varying depth of tissue necrosis even if the source of energy is constant.21-25 Similarly, varying the light source, and wavelengths, will result in varying penetration into esophageal tissue. For example, red light (630 nm) seems to penetrate esophageal mucosa deeper than green light (532 nm).
Lastly, one needs to understand the ablative modalities used in a given study. Most regulatory trials allow the use of PDT only. On the other hand, clinical series seem to use additional ablative modalities, like radiofrequency energy or argon plasma coagulation (APC), to ablate residual BE after the initial PDT treatment. Such use, though appropriate,26 may introduce bias into the final conclusions of a study unless clearly accounted for by the authors.
Dr. Norman Barrett initially described BE, intestinal metaplasia of the esophagus, in the 1950s. Since then, the link between BE and esophageal adenocarcinoma has been extensively studied and well established.27,28 The incidence of esophageal cancer is on the rise.29,30 The development of esophageal adenocarcinoma among patients with BE seems to follow an established pathway through LGD, HGD, then adenocarcinoma. Therefore, detecting and treating dysplasia is of critical importance. Yet, dysplasia can be hard to detect in white light endoscopy (WLE). Therefore, a protocol was established to perform four-quadrant biopsy every 1 to 2 cm of BE (Seattle protocol). This, however, is cumbersome, and many clinicians do not adhere to such protocol resulting in many false negative screening. Therefore, many studies have evaluated the use of chromoendoscopy (CE) to detect dysplasia in BE. CE uses various dyes (indigo carmine, methylene blue, acetic acid, etc.) to highlight vascular patterns on surface mucosa. This enables the endoscopist to perform targeted biopsies of suspicious areas and increases the yield for dysplasia. Virtual chromoendoscopy (VE) uses various light sources and lenses on the endoscope in order to highlight the surface vasculature.
Both methods have been extensively studied in patients with BE. We recently assessed the increase in the diagnostic yield in detecting dysplasia using VE and CE compared to WLE with random biopsies in a meta-analysis. The study has been submitted for publication and an abstract form has been submitted to Digestive Disease Week 2013. We found that using either CE or VE increases the yield of detecting dysplasia by 34% (95% confidence interval, 20% to 56%, p=0.0001). This study, among others, highlights the importance of improved endoscopic imaging, which will be required to detect and treat patients with Barrett's dysplasia.
As discussed earlier, PDT offers different tissue penetration depending on the agent and the wavelength of the light source. In the esophagus, PDT offers clear advantages by allowing targeted therapy to the affected area using endoscopy. By controlling the depth of tissue necrosis, one can ensure eradication of Barrett's mucosa while minimizing the risk of esophageal perforation.31
In early clinical studies, surgeons and gastroenterologists used Ps combined with red light for palliation of obstructing lesions, as well as for curative treatment of early cancers. As clinicians became more comfortable with the technique, and with continued improvements in endoscopic light dosimetry, the uses of sodium porfimer PDT expanded to esophageal dysplasia (BE and squamous disease). As a result, Ps has been extensively studied in the United States and North America. The efficacy of this form of PDT in eradicating esophageal dysplasia has been documented in large prospective studies.5,12
Ps, however, is a first generation agent that has some shortcomings. Ps is not specific to mucosa. In addition, it causes prolonged photosensitivity, lasting up to 6 weeks, which can cause significant skin damage if a patient is exposed to light. Therefore, new photosensitizers have been produced to overcome those issues. One of such agents is metatetrahydroxy-phenyl chlorine (mTHPC, temoporfin, Foscan; Biolitec AG, Jena, Germany). The mTHPC is more potent compared to sodium porfimer, requires a smaller dosage and lower light energy. The mTHPC shortens the period of photosensitivity to 2 to 3 weeks. Yet, it seems to be associated with an increased risk of perforation and stricture formation compared to sodium porfimer.32 ALA is another photosensitizer which works on mucosal cells only.33 ALA allows targeted necrosis of mucosal cells only, thus preventing damage to submucosal tissue. In theory, this should lead to decreased risk of perforation and stricture formation.25 A recent trial from University College London sought to randomize 128 patients with Barrett's HGD to PDT using 5-ALA or Ps. Over the study period from 2006 to 2009, 64 patients were actually randomized to PDT with complete response for HGD in 16/34 patients in the ALA group (47%) and 12/30 patients in the Ps group (40%). The overall cancer incidence was 14% (nine/64 patients) that was usually treated with additional PDT or EMR plus chemo-radiation therapy. At a median 2 year follow-up, there was no statistically significant difference in outcome of PDT using either 5-ALA or Ps. A subgroup analysis suggested that patients with BE segments less than 6 cm length had improved outcomes with low risk of stricture or death after 5-ALA PDT.34 Several other agents have been studied as photosensitizers in PDT. Those are summarized in Table 1, after Yano et al.20
As mentioned earlier, Ps is the most widely used agent for esophageal PDT. It is administered by intravenous injection. This chemical is a HpD and was first approved in the United States in 1995 for use among patient with advanced esophageal carcinoma.35 The best study on sodium porfimer PDT came from Overholt et al.35 who introduced cylindrical inflatable balloon which is used to administer light to 101 patients with HGD. Patients were then followed up for 4 years showing a resolution of HGD in 54% complete remission of intestinal metaplasia (CRIM). Among patients with LGD, success rate was 93%. The success rate for HGD and cancer were noted to be 78% and 48%. In those patients, stenosis was noted in 30% of all patients. A larger (208 patients), multicenter study by Overholt et al.35 later showed complete eradication of HGD in 77% versus 39% among patients who had PDT and control respectively. This response was still present at 5 years of follow-up.
Since then, several other centers have reported clinical results of PDT. We reported our experience in 102 patients with BE and HGD/EC.11,36-38 After a mean follow-up of 18 months, a single session of Ps PDT showed CRIM in 56% of patients.38 We also reviewed the combined experience of Mayo Clinic in Jacksonville and Rochester. One hundred and forty-two patients with BE and HGD were treated with sodium porfimer PDT. At 19 months of follow-up, CRIM was noted in 50% of patients in Jacksonville compared to 35% in Rochester.39,41 HGD was eliminated in 100% in Jacksonville, and 80% in Rochester. Esophageal strictures were noted in 20% of patients in Jacksonville and 27% of patients in Rochester.
Stricture formation is one of the most common complications post PDT. Panjehpour et al.41,42 looked at the effect of oral steroids post PDT on stricture formation. Sixty patients with BE and HGD were randomized to be treated with either PDT alone or PDT combined with oral prednisone. Surprisingly, the results showed a trend towards more stricture formation in the prednisone group compared (29% vs. 16%); yet this did not reach statistical significance.41,42
As previously mentioned, ALA is another photosensitizing agent that has been used in PDT. ALA is a pro-drug which stimulates the endogenous production of protoporphyrin IX within the gut mucosa.43,44 ALA compounds have been used in Europe of several years. A commercially available form of ALA, Levulan, was approved by the US Food and Drug Administration.45 ALA is usually activated by red light and was expected to offer several advantages including a shorter photosensitivity period of 1 to 2 days and the preferential targeting of the superficial mucosal layer.46-49
The initial studies on ALA PDT were encouraging.50-53 Most notably, Ackroyd et al.54,55 conducted a double-blinded, randomized, placebo-controlled clinical trial among patients with BE and LGD using ALA PDT. After a mean follow-up of 24 months, complete remission of dysplasia was achieved in 98% of patients.54,55
Pech et al.56 used ALA PDT to treat 35 patients with BE with HGD and reported a very high complete response rate of 97% of patients after mean follow-up of 42 months. Since then, several other studies have been published with similar results.56-59
Despite the encouraging results of those studies, orally-administered ALA was found to be associated with significant side effects including elevated liver enzyme tests, chest pain, neuropathy, and sudden death.60 To avoid such toxicity, Ortner et al.61 administered ALA topically using a spray catheter at the time of endoscopy. This was used to treat seven patient with BE and seven patients with BE and LGD.61 The results of this study showed progression from BE to HGD in one patient and CRIM in only 21% of patients. Hence, this form of using ALA was abandoned. As discussed above, at a median 2 year follow-up, there was no statistically significant difference in outcome of PDT using either 5-ALA or Ps. However, a subgroup analysis suggested that patients with BE segments less than 6 cm length had improved outcomes with low risk of stricture or death after 5-ALA PDT.34
In addition to the above shortcomings, ALA PDT appears to perform poorly when compared to other ablation methods. In a study by Ragunath et al.,62 ALA PDT was compared to APC in 26 patients with BE with dysplasia. The authors found that APC was more effective, safer, and less expensive compared to ALA PDT. Other authors have reproduced these results.54,63,64 Therefore, the correct ALA dose and the optimal light dose have not been established and further studies need to be conducted before this form of PDT can be recommended as first line for Barrett's ablation.
As discussed earlier, mTHPC, a chlorine derivative, is a potent photosensitizer. It has a shorter period of photosensitivity compared to sodium porfimer and requires less light and lower drug doses to produce the effect. The use of mTHPC has been limited due to reported cases of tissue necrosis among patients with head and neck cancer.65 The use of mTHPC in patients with BE has been limited to a small number of studies (Table 2). The optimal light settings and drug dosimetry are not known. Further studies may be needed to optimize treatment parameters for mTHPC PDT in patients with BE.
At its core, PDT works by sensitizing esophageal mucosa to light. Therefore, the method by which light is delivered is of great importance to the success of this therapy. There is no easy way to determine the ideal dosage of light for any given patients. Too much light may lead to tissue necrosis and stricture formation. Too little light means incomplete eradication of dysplasia or BE. In addition to finding the right dosage, one has to overcome the uneven exposure to light caused by esophageal folds. Due to peristalsis and respiratory motion, maintaining central positioning of the light sources can be a challenge.50 The light delivery system is usually composed of a modified diffuser. One way to overcome this was the creation of an elastic catheter balloon.66 This allowed better centering the fiber and more equal distribution of light to the treatment area. However, over-distention of the esophageal wall may result in decreased mucosal perfusion leading to decreasing effectiveness.66,67 In addition to the balloon, another group of researchers used a large diameter rigid light distributor to improve light delivery.68 The use of advanced optical techniques, such as fluorescence spectroscopy or optical coherence tomography, may help assess drug levels at the level of the mucosa and the progression of the photodynamic reaction to improve PDT outcomes.69-71 The development of improved light dosimetry and delivery system will be crucial to the future of PDT and will determine if PDT can remain a viable endoscopic mucosal ablation treatment option.72-74
PDT has been particularly promising in patients with advanced esophageal carcinoma that was unsuitable for surgery, or for persistent/recurrent disease after chemoradiation therapy (CRT). Yano et al.20 performed Ps-PDT as salvage treatment in patients with recurrent disease after CRT with promising results. Subsequently, a phase I/II study is being conducted using PDT with talaporfin sodium in patients with recurrent disease after CRT for esophageal carcinoma in Japan.20 Lindenmann has also reported the salvage or palliative use of Ps PDT to prolong survival in patients with persistent/recurrent disease after CRT for esophageal cancer.75
EMR uses diathermy snare to resect nodular, dysplastic, or neoplastic esophageal lesions. EMR is used for staging of esophageal neoplasia but can also be used for curative intent.76 There are several EMR techniques. The most common includes using the multiband mucosectomy method with or without saline injection lift to remove the most suspicious lesions (DT-6-5F; Cook Medical, Bloomington, IN, USA). This involves placement of a rubber band around the suspicious lesion followed by resection of the area using a snare. For larger lesions, the area is lifted by injection of fluid, which can vary by different endoscopists. A cap is used to suction the mucosa while a snare is used to resect the affected area (K-008; Olympus America Inc., Center Valley, PA, USA). This is repeated multiple times until the whole lesion has been resected.
Several studies have established the utility of EMR, with or without ablation therapy, for treatment of EC and HGD in patients with BE.77-80 When EMR is done on focal lesions, Barrett's mucosa is still present and dysplasia or neoplasia can recur. There are two schools of thought regarding the eradication of BE. The traditional approach has been to use ablation after EMR with the goal of achieving CRIM.
Complete Barrett's eradication is a subset of EMR in which the whole BE mucosa is resected endoscopically. In doing so, there is no need for ablative therapies including PDT. Satodate et al.81 first described this approach as a case report in 2003. Since then, other investigators have studied and advocated for this approach. Using this approach, studies have shown the rate of CRIM to be 86% to 100%. The main limitation for this approach is the high rate of dysphagia due to stenosis (up to 50%). This approach continues to be a viable alternative to patients who do not undergo ablative therapies including PDT.80,82-84
Besides PDT, other ablative therapies are used for this purpose. This includes radiofrequency ablation (RFA), cryotherapy, and APC.85-87 The most commonly used of those is RFA using the HALO system (MARRX Medical Inc., Sunnyvale, CA, USA). RFA can be applied locally or circumferentially. Under standard settings, the depth of penetration is 500 to 1,000 µm. The most important study on RFA came by Shaheen et al.87 This was a multicentered, randomized, controlled clinical trial of 127 patients with Barrett's dysplasia. Patients were randomized to RFA or sham endoscopic therapy in a 2:1 ratio. The final results showed CRIM in 77.4% of RFA group compared to 2.3% in the control group (p<0.0001). Progression to cancer was seen in 1.2% in the RFA group compared to 9.3% in the control group (p<0.05). In addition, RFA has had a low rate of stenosis of less than 3% and perforation seems to occur in less than 0.2% of patients.88,89
For many years, PDT became an important method for mucosal ablation in patients with BE and squamous dysplasia and carcinoma. Ps PDT has been shown to be a safe and reliable treatment method for eliminating dysplasia and preventing the development of esophageal cancer. The technique seems to have the advantage of selectively targeting mucosal layer while minimizing stricturing and perforation. However, significant drawbacks to PDT have caused a decrease in the use of this method compared to RFA. These include the need for IV administration agents and extended periods of photosensitization. Newer photosensitizing agents and more sophisticated light dosimetry will be needed to bring PDT back to mainstream ablative therapy in gastroenterology.
References
1. Hahn SM, Putt ME, Metz J, et al. Photofrin uptake in the tumor and normal tissues of patients receiving intraperitoneal photodynamic therapy. Clin Cancer Res. 2006; 12:5464–5470. PMID: 17000681.

2. McCaughan JS Jr, Ellison EC, Guy JT, et al. Photodynamic therapy for esophageal malignancy: a prospective twelve-year study. Ann Thorac Surg. 1996; 62:1005–1009. PMID: 8823080.

3. Litle VR, Luketich JD, Christie NA, et al. Photodynamic therapy as palliation for esophageal cancer: experience in 215 patients. Ann Thorac Surg. 2003; 76:1687–1692. PMID: 14602313.

4. Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005; 62:488–498. PMID: 16185958.

5. Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc. 2007; 66:460–468. PMID: 17643436.

6. Wolfsen HC. Endoluminal therapy for Barrett's esophagus. Gastrointest Endosc Clin N Am. 2007; 17:59–82. PMID: 17397777.

7. Wolfsen HC. Present status of photodynamic therapy for high-grade dysplasia in Barrett's esophagus. J Clin Gastroenterol. 2005; 39:189–202. PMID: 15718860.

8. Wolfsen HC. Carpe luz-seize the light: endoprevention of esophageal adenocarcinoma when using photodynamic therapy with porfimer sodium. Gastrointest Endosc. 2005; 62:499–503. PMID: 16185960.

9. Wolfsen HC. Endoprevention of esophageal cancer: endoscopic ablation of Barrett's metaplasia and dysplasia. Expert Rev Med Devices. 2005; 2:713–723. PMID: 16293098.

10. Prosst RL, Wolfsen HC, Gahlen J. Photodynamic therapy for esophageal diseases: a clinical update. Endoscopy. 2003; 35:1059–1068. PMID: 14648421.

11. Wang KK. Current status of photodynamic therapy of Barrett's esophagus. Gastrointest Endosc. 1999; 49(3 Pt 2):S20–S23. PMID: 10049443.

12. Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett's esophagus with dysplasia and/or early stage carcinoma: long-term results. Gastrointest Endosc. 2003; 58:183–188. PMID: 12872083.

13. Sampliner RE. Prevention of adenocarcinoma by reversing Barrett's esophagus with mucosal ablation. World J Surg. 2003; 27:1026–1029. PMID: 12917762.

14. Winship MJ, Wang S, Chen JC, Keltner L, Christophersen JS. Treatment of colorectal liver metastases using talaporfin sodium and intratumoral placement of LEDs [abstract]. J Clin Oncol. 2005; 23(16 Suppl):3663. PMID: 15738543.
15. Konan-Kouakou YN, Boch R, Gurny R, Allémann E. In vitro and in vivo activities of verteporfin-loaded nanoparticles. J Control Release. 2005; 103:83–91. PMID: 15710502.

16. Dougherty TJ. An update on photodynamic therapy applications. J Clin Laser Med Surg. 2002; 20:3–7. PMID: 11902352.

17. Allison SP, Lobo DN. Fluid and electrolytes in the elderly. Curr Opin Clin Nutr Metab Care. 2004; 7:27–33. PMID: 15090900.
18. Qiang YG, Zhang XP, Li J, Huang Z. Photodynamic therapy for malignant and non-malignant diseases: clinical investigation and application. Chin Med J (Engl). 2006; 119:845–857. PMID: 16732988.

19. Pereira SP, Ayaru L, Ackroyd R, et al. The pharmacokinetics and safety of porfimer after repeated administration 30-45 days apart to patients undergoing photodynamic therapy. Aliment Pharmacol Ther. 2010; 32:821–827. PMID: 20629974.
20. Yano S, Hirohara S, Obata M, et al. Current states and future views in photodynamic therapy. J Photochem Photobiol C. 2011; 12:46–67.

21. Perry Y, Epperly MW, Fernando HC, et al. Photodynamic therapy induced esophageal stricture: an animal model: from mouse to pig. J Surg Res. 2005; 123:67–74. PMID: 15652952.
22. Cramers P, Ruevekamp M, Oppelaar H, Dalesio O, Baas P, Stewart FA. Foscan uptake and tissue distribution in relation to photodynamic efficacy. Br J Cancer. 2003; 88:283–290. PMID: 12610515.

23. Wagnieres G, Hadjur C, Grosjean P, et al. Clinical evaluation of the cutaneous phototoxicity of 5,10,15,20-tetra(m-hydroxyphenyl)chlorin. Photochem Photobiol. 1998; 68:382–387. PMID: 9747593.
24. Barr H, Kendall C, Stone N. Photodynamic therapy for esophageal cancer: a useful and realistic option. Technol Cancer Res Treat. 2003; 2:65–76. PMID: 12625755.

25. Bown SG, Rogowska AZ. New photosensitizers for photodynamic therapy in gastroenterology. Can J Gastroenterol. 1999; 13:389–392. PMID: 10377468.

26. Paskowitz DM, Donohue-Rolfe KM, Yang H, et al. Neurotrophic factors minimize the retinal toxicity of verteporfin photodynamic therapy. Invest Ophthalmol Vis Sci. 2007; 48:430–437. PMID: 17197564.

27. Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001; 32:368–378. PMID: 11331953.

28. Zaninotto G, Minnei F, Guirroli E, et al. The Veneto region's Barrett's oesophagus registry: aims, methods, preliminary results. Dig Liver Dis. 2007; 39:18–25. PMID: 17141593.

30. Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998; 83:2049–2053. PMID: 9827707.

31. Tokar JL, Haluszka O, Weinberg DS. Endoscopic therapy of dysplasia and early-stage cancers of the esophagus. Semin Radiat Oncol. 2007; 17:10–21. PMID: 17185193.

32. Andrejevic Blant S, Grosjean P, Ballini JP, et al. Localization of tetra(m-hydroxyphenyl) chlorin (Foscan) in human healthy tissues and squamous cell carcinomas of the upper aero-digestive tract, the esophagus and the bronchi: a fluorescence microscopy study. J Photochem Photobiol B. 2001; 61:1–9. PMID: 11485842.
33. Ackroyd R, Kelty C, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001; 74:656–669. PMID: 11723793.

34. Dunn JM, Mackenzie GD, Banks MR. A randomised controlled trial of ALA vs. Photofrin photodynamic therapy for high-grade dysplasia arising in Barrett's oesophagus. Lasers Med Sci;Epub 2012 Jun 15. DOI: http://dx.doi.org/10.1007/s10103-012-1132-1.
35. Overholt B, Panjehpour M, Tefftellar E, Rose M. Photodynamic therapy for treatment of early adenocarcinoma in Barrett's esophagus. Gastrointest Endosc. 1993; 39:73–76. PMID: 8454152.

36. Wang KK, Kim JY. Photodynamic therapy in Barrett's esophagus. Gastrointest Endosc Clin N Am. 2003; 13:483–489. PMID: 14629104.

37. Wolfsen HC, Woodward TA, Raimondo M. Photodynamic therapy for dysplastic Barrett esophagus and early esophageal adenocarcinoma. Mayo Clin Proc. 2002; 77:1176–1181. PMID: 12440553.

38. Wolfsen HC, Hemminger LL, Wallace MB, Devault KR. Clinical experience of patients undergoing photodynamic therapy for Barrett's dysplasia or cancer. Aliment Pharmacol Ther. 2004; 20:1125–1131. PMID: 15569115.

39. Wang KK, Wong Kee, Buttar N, Papenfuss S, Lutzke L. Barrett's esophagus after photodynamic therapy: risk of cancer development during long term follow-up [abstract]. Gastroenterology. 2004; 126(5 Suppl 2):A50.
40. Wolfsen HC, Hemminger LL. Photodynamic therapy for dysplastic Barrett's esophagus and mucosal adenocarcinoma [abstract]. Gastrointest Endosc. 2004; 59:AB251.
41. Panjehpour M, Overholt BF, Haydek JM, Lee SG. Results of photodynamic therapy for ablation of dysplasia and early cancer in Barrett's esophagus and effect of oral steroids on stricture formation. Am J Gastroenterol. 2000; 95:2177–2184. PMID: 11007214.

42. Panjehpour M, Overholt BF, Phan MN, Haydek JM. Optimization of light dosimetry for photodynamic therapy of Barrett's esophagus: efficacy vs. incidence of stricture after treatment. Gastrointest Endosc. 2005; 61:13–18. PMID: 15672050.

43. Peng Q, Warloe T, Moan J, et al. Antitumor effect of 5-aminolevulinic acid-mediated photodynamic therapy can be enhanced by the use of a low dose of photofrin in human tumor xenografts. Cancer Res. 2001; 61:5824–5832. PMID: 11479222.
44. Peng Q, Warloe T, Berg K, et al. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer. 1997; 79:2282–2308. PMID: 9191516.
45. Godal A, Nilsen NO, Klaveness J, Branden JE, Nesland JM, Peng Q. New derivatives of 5-aminolevulinic acid for photodynamic therapy: chemical synthesis and porphyrin production in in vitro and in vivo biological systems. J Environ Pathol Toxicol Oncol. 2006; 25:109–126. PMID: 16566712.

46. Bedwell J, MacRobert AJ, Phillips D, Bown SG. Fluorescence distribution and photodynamic effect of ALA-induced PP IX in the DMH rat colonic tumour model. Br J Cancer. 1992; 65:818–824. PMID: 1616853.

47. Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992; 14:275–292. PMID: 1403373.
48. Peng Q, Berg K, Moan J, Kongshaug M, Nesland JM. 5-Aminolevulinic acid-based photodynamic therapy: principles and experimental research. Photochem Photobiol. 1997; 65:235–251. PMID: 9066303.

49. Webber J, Kessel D, Fromm D. Side effects and photosensitization of human tissues after aminolevulinic acid. J Surg Res. 1997; 68:31–37. PMID: 9126192.

50. Gossner L, May A, Sroka R, Ell C. A new long-range through-the-scope balloon applicator for photodynamic therapy in the esophagus and cardia. Endoscopy. 1999; 31:370–376. PMID: 10433046.

51. Gossner L, Stolte M, Sroka R, et al. Photodynamic ablation of high-grade dysplasia and early cancer in Barrett's esophagus by means of 5-aminolevulinic acid. Gastroenterology. 1998; 114:448–455. PMID: 9496934.

52. Barr H, Shepherd NA, Dix A, Roberts DJ, Tan WC, Krasner N. Eradication of high-grade dysplasia in columnar-lined (Barrett's) oesophagus by photodynamic therapy with endogenously generated protoporphyrin IX. Lancet. 1996; 348:584–585. PMID: 8774572.

53. Orth K, Stanescu A, Rück A, Russ D, Beger HG. Photodynamic ablation and argon-plasma coagulation of premalignant and early-stage malignant lesions of the oesophagus: an alternative to surgery? Chirurg. 1999; 70:431–438. PMID: 10354840.
54. Ackroyd R, Brown NJ, Davis MF, et al. Photodynamic therapy for dysplastic Barrett's oesophagus: a prospective, double blind, randomised, placebo controlled trial. Gut. 2000; 47:612–617. PMID: 11034574.

55. Ackroyd R, Brown NJ, Davis MF, Stephenson TJ, Stoddard CJ, Reed MW. Aminolevulinic acid-induced photodynamic therapy: safe and effective ablation of dysplasia in Barrett's esophagus. Dis Esophagus. 2000; 13:18–22. PMID: 11005326.

56. Pech O, Gossner L, May AD, Stolte M, Ell C. Long term results of PDT for early neoplasia in Barrett's esophagus [abstract]. Gastrointest Endosc. 2004; 59:P257.
57. Guelrud M, Herrera I, Essenfeld H, Castro J. Enhanced magnification endoscopy: a new technique to identify specialized intestinal metaplasia in Barrett's esophagus. Gastrointest Endosc. 2001; 53:559–565. PMID: 11323579.

58. Behrens A, May A, Gossner L, et al. Curative treatment for high-grade intraepithelial neoplasia in Barrett's esophagus. Endoscopy. 2005; 37:999–1005. PMID: 16189774.

59. May A, Gossner L, Pech O, et al. Intraepithelial high-grade neoplasia and early adenocarcinoma in short-segment Barrett's esophagus (SSBE): curative treatment using local endoscopic treatment techniques. Endoscopy. 2002; 34:604–610. PMID: 12173079.

60. Sylantiev C, Schoenfeld N, Mamet R, Groozman GB, Drory VE. Acute neuropathy mimicking porphyria induced by aminolevulinic acid during photodynamic therapy. Muscle Nerve. 2005; 31:390–393. PMID: 15490483.

61. Ortner MA, Zumbusch K, Liebetruth J, et al. Is topical delta-aminolevulinic acid adequate for photodynamic therapy in Barrett's esophagus? A pilot study. Endoscopy. 2002; 34:611–616. PMID: 12173080.
62. Ragunath K, Krasner N, Raman VS, Haqqani MT, Phillips CJ, Cheung I. Endoscopic ablation of dysplastic Barrett's oesophagus comparing argon plasma coagulation and photodynamic therapy: a randomized prospective trial assessing efficacy and cost-effectiveness. Scand J Gastroenterol. 2005; 40:750–758. PMID: 16118910.

63. Kelty CJ, Ackroyd R, Brown NJ, Brown SB, Reed MW. Comparison of high- vs low-dose 5-aminolevulinic acid for photodynamic therapy of Barrett's esophagus. Surg Endosc. 2004; 18:452–458. PMID: 14752635.
64. Kelty CJ, Ackroyd R, Brown NJ, Stephenson TJ, Stoddard CJ, Reed MW. Endoscopic ablation of Barrett's oesophagus: a randomized-controlled trial of photodynamic therapy vs. argon plasma coagulation. Aliment Pharmacol Ther. 2004; 20:1289–1296. PMID: 15606390.

65. Andrejevic-Blant S, Hadjur C, Ballini JP, et al. Photodynamic therapy of early squamous cell carcinoma with tetra(m-hydroxyphenyl)chlorin: optimal drug-light interval. Br J Cancer. 1997; 76:1021–1028. PMID: 9376261.

66. van den Bergh H. On the evolution of some endoscopic light delivery systems for photodynamic therapy. Endoscopy. 1998; 30:392–407. PMID: 9689515.

67. Overholt BF, Panjehpour M, DeNovo RC, Peterson MG, Jenkins C. Balloon photodynamic therapy of esophageal cancer: effect of increasing balloon size. Lasers Surg Med. 1996; 18:248–252. PMID: 8778519.

68. Stepinac T, Grosjean P, Woodtli A, Monnier P, van den Bergh H, Wagnieres G. Optimization of the diameter of a radial irradiation device for photodynamic therapy in the esophagus. Endoscopy. 2002; 34:411–415. PMID: 11972275.

69. Braichotte DR, Savary JF, Monnier P, van den Bergh HE. Optimizing light dosimetry in photodynamic therapy of early stage carcinomas of the esophagus using fluorescence spectroscopy. Lasers Surg Med. 1996; 19:340–346. PMID: 8923430.

70. Zellweger M, Grosjean P, Monnier P, van den Bergh H, Wagnières G. Stability of the fluorescence measurement of Foscan in the normal human oral cavity as an indicator of its content in early cancers of the esophagus and the bronchi. Photochem Photobiol. 1999; 69:605–610. PMID: 10333768.

71. Standish BA, Yang VX, Munce NR, et al. Doppler optical coherence tomography monitoring of microvascular tissue response during photodynamic therapy in an animal model of Barrett's esophagus. Gastrointest Endosc. 2007; 66:326–333. PMID: 17643708.
72. Boere IA, Robinson DJ, de Bruijn HS, et al. Monitoring in situ dosimetry and protoporphyrin IX fluorescence photobleaching in the normal rat esophagus during 5-aminolevulinic acid photodynamic therapy. Photochem Photobiol. 2003; 78:271–277. PMID: 14556314.

73. Cheung R, Solonenko M, Busch TM, et al. Correlation of in vivo photosensitizer fluorescence and photodynamic-therapy-induced depth of necrosis in a murine tumor model. J Biomed Opt. 2003; 8:248–252. PMID: 12683850.

74. Radu A, Conde R, Fontolliet C, Wagnieres G, Van den Bergh H, Monnier P. Mucosal ablation with photodynamic therapy in the esophagus: optimization of light dosimetry in the sheep model. Gastrointest Endosc. 2003; 57:897–905. PMID: 12776039.

75. Lindenmann J, Matzi V, Neuboeck N, et al. Individualized, multimodal palliative treatment of inoperable esophageal cancer: clinical impact of photodynamic therapy resulting in prolonged survival. Lasers Surg Med. 2012; 44:189–198. PMID: 22334351.

76. Namasivayam V, Wang KK, Prasad GA. Endoscopic mucosal resection in the management of esophageal neoplasia: current status and future directions. Clin Gastroenterol Hepatol. 2010; 8:743–754. PMID: 20541628.

77. Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's esophagus. Gastroenterology. 2000; 118:670–677. PMID: 10734018.

78. Pech O, Bollschweiler E, Manner H, Leers J, Ell C, Hölscher AH. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett's esophagus at two high-volume centers. Ann Surg. 2011; 254:67–72. PMID: 21532466.

79. Moss A, Bourke MJ, Hourigan LF, et al. Endoscopic resection for Barrett's high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010; 105:1276–1283. PMID: 20179694.

80. Larghi A, Lightdale CJ, Ross AS, et al. Long-term follow-up of complete Barrett's eradication endoscopic mucosal resection (CBE-EMR) for the treatment of high grade dysplasia and intramucosal carcinoma. Endoscopy. 2007; 39:1086–1091. PMID: 17701854.

81. Satodate H, Inoue H, Yoshida T, et al. Circumferential EMR of carcinoma arising in Barrett's esophagus: case report. Gastrointest Endosc. 2003; 58:288–292. PMID: 12872107.

82. Chung A, Bourke MJ, Hourigan LF, et al. Complete Barrett's excision by stepwise endoscopic resection in short-segment disease: long term outcomes and predictors of stricture. Endoscopy. 2011; 43:1025–1032. PMID: 22068701.

83. Chennat J, Konda VJ, Ross AS, et al. Complete Barrett's eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma: an American single-center experience. Am J Gastroenterol. 2009; 104:2684–2692. PMID: 19690526.
84. Peters FP, Krishnadath KK, Rygiel AM, et al. Stepwise radical endoscopic resection of the complete Barrett's esophagus with early neoplasia successfully eradicates pre-existing genetic abnormalities. Am J Gastroenterol. 2007; 102:1853–1861. PMID: 17509033.

85. Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer). Gastrointest Endosc. 2007; 65:3–10. PMID: 17185072.

86. Sampliner RE. Endoscopic therapy for Barrett's esophagus. Clin Gastroenterol Hepatol. 2009; 7:716–720. PMID: 19306943.

87. Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology. 2011; 141:460–468. PMID: 21679712.
88. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy. 2010; 42:781–789. PMID: 20857372.

89. Lyday WD, Corbett FS, Kuperman DA, et al. Radiofrequency ablation of Barrett's esophagus: outcomes of 429 patients from a multicenter community practice registry. Endoscopy. 2010; 42:272–278. PMID: 20146164.

Table 1
Photosensitizers for Esophageal Photodynamic Therapy
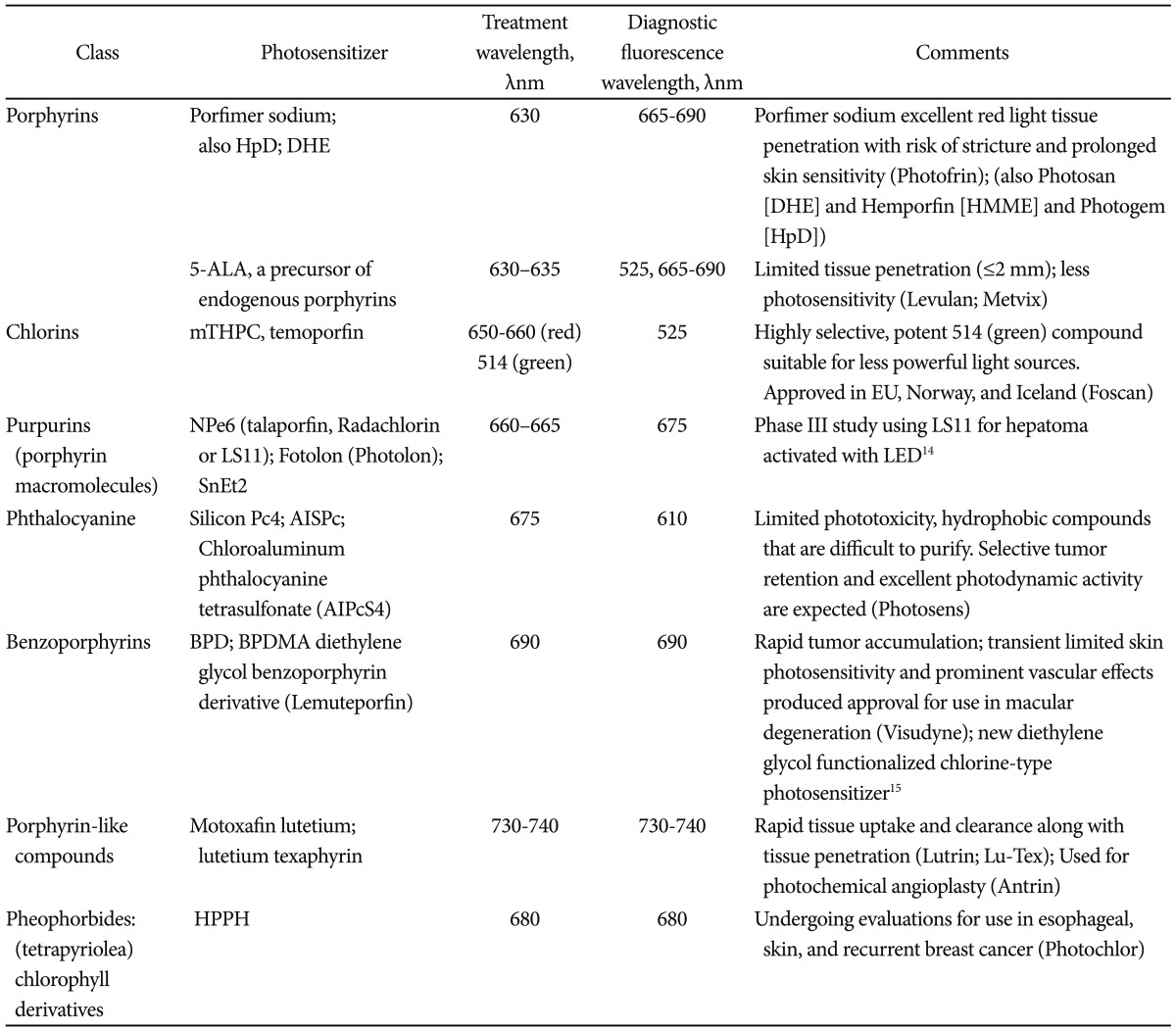
HpD, hematoporphyrin derivative; DHE, dihematoporphyrin ether; HMME, hematoporphyrin monomethyl ether; ALA, aminolevulinic acid; mTHPC, meso-tetrahydroxyphenyl chlorine; NPe6, mono-L-aspartyl chlorine e6; SnEt2, tin-etio-purpurin; LED, light emitting diode; Pc4, phthalocyanine; AISPc, aluminum disulphonated phthalocyanine; AIPcS4, aluminum phthalocyanine tetrasulfonate; BPD, benzoporphyrin derivative; BPDMA, benzoporphyrin derivative monoacid; HPPH, 2-[1-hexyloxyethyl]2-devinyl-pyropheophorbide-a.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download