Abstract
With the increasing interest in endoscopy and the rising number of endoscopic examinations in hospitals, the importance of endoscopic reprocessing is also increasing. Cure facilities that are understaffed and ill-equipped are trying to cope with the problems of insufficient cleaning and high infection risks. To prevent endoscopy-associated infection, the endoscope cleaning, and disinfection guidelines prepared by the Korean Society of Gastrointestinal Endoscopy must be followed. In this review, the steps of endoscopic reprocessing and the equipments required in each step are discussed.
Go to : 
The development of gastrointestinal endoscopic instruments and the National Cancer Screening Program have led to growing interest in and the performance of endoscopy and the early detection of gastrointestinal diseases. Enhanced quality of endoscopic examination and disinfection is required. During the endoscopic examination, endoscope, and its accessories are inserted into the body, so they may play the role of viral and bacterial transmission paths of infection. In particular, the risk of infection substantially increases when endoscope and its accessories are reused. In an effort to prevent endoscopic examination-mediated transmission paths of infection, Korean Society of Gastrointestinal Endoscopy established endoscope cleaning and disinfection guidelines in 1995.1 The guidelines were revised in November 2009 and August 2012 (Table 1).2 In this review, the endoscopic reprocessing steps and their required equipment are discussed.
Go to : 
Sterilization involves complete removal of all types of living microorganisms. The methods of sterilization include high-pressure steam sterilization, gas sterilization, and chemical sterilization. The types of disinfection include high-, intermediate-, and low-level disinfection. High-level disinfection kills all microorganisms except some bacterial spores. Intermediate-level disinfection removes mycobacteria, vegetative bacteria, most viruses, and fungi, except bacterial spores. Low-level disinfection removes most vegetative bacteria and some fungi.
Most infections during endoscopic examinations are caused by bacteria, followed by viruses, fungi, protozoas, and prions. The routes of infection include nonendoscopic routes such as saline solutions, injection solutions, and endoscopy treatment room environments, and endoscopic routes. Endoscopy-associated infections are divided into endogenous infections and exogenous infections. Endogenous infection occurs when the microorganisms in the patient's respiratory tract or gastrointestinal tract are moved to patient's blood or another organs via the endoscope.3 Exogenous infection is caused by a contaminated endoscope or its accessory due to its inappropriate cleaning and disinfection.4,5 Few cases of endoscopy-associated infection have been reported in South Korea. Between 1966 and 1992, 281 such cases were reported in the USA.6 According to the report of the American Society of Gastrointestinal Endoscopy in 1993, the frequency of endoscopy-associated infection cases was estimated to be 1.8 million to 1 during the period between 1988, when the endoscope disinfection guidelines were introduced, and 1992.7 If clinicians abide by the current disinfection guidelines in endoscopy, the risk of endoscopy-associated infection may be significantly lowered.
Go to : 
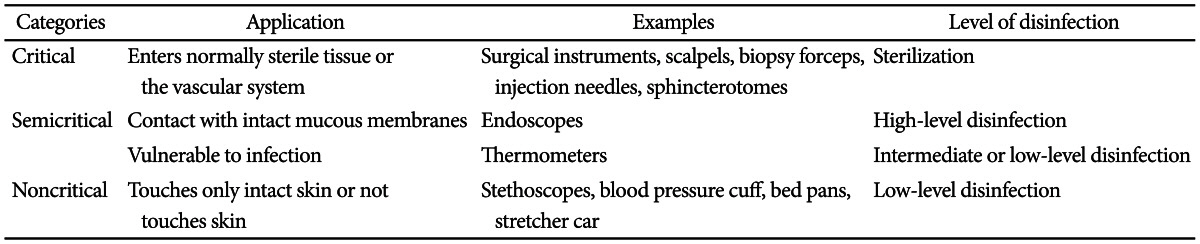
Endoscopic devices have different risks of infection (Table 2) and require different levels of bacterial removal according to their purposes and types. Spaulding suggested different levels of infection risks in medical devices.7
This level is required for medical devices that are inserted into the sterile tissues or the vascular system, such as biopsy forceps, injection needles, and sphincterotomes. Sterilization is recommended.8
This level is required for medical devices that come in contact with the mucous membrane, such as endoscopes. A high-level disinfection level is recommended.9
This level is required for medical devices that do not directly come in contact with patients or come in contact only with their skin, such as stretcher car, stethoscopes and blood pressure cuff. These devices can be cleaned with soap and water. A low-level disinfection is recommended.
Go to : 
There are two methods of endoscopic reprocessing: by hand and by using automated endoscope reprocessor. The reprocessing steps consist of precleaning, cleaning, disinfection, rinsing, and drying. For the reprocessing, clinicians, nurses, and cleaning staff must use individual protection equipment to protect themselves from contaminants and to reduce their exposure to chemicals such as disinfection solutions. Protective devices such as gloves, waterproof gowns, masks, and goggles must always be available and usable.10
Necessary materials: enzymatic detergent or neutral detergent; disposable, disinfected or sterilized fabric or sponge; and carrying case with cover.
This is the first step of the endoscopic reprocessing procedure. Immediately after the endoscopic examination beside the bed, foreign objects on the surface of the endoscope are removed using a fabric or sponge soaked in an enzymatic detergent or a neutral detergent. A disposable fabric or sponge is desirable. If both are unavailable, the replacement must be disinfected or sterilized.11 The endoscope fore-end is put in the cleansing solution, and air is repeatedly blown in and out of the endoscope until the solution becomes clean. Due to the biofilm that is formed on the inner wall of the extension pipe of the endoscope, the effect of the disinfection is compromised. Water irrigation must be performed before the contaminants dry up and stick to the biopsy channel. Once the contaminants coagulate, they could not be completely removed by any disinfection procedure. Finally, air is sucked in before the endoscope is unplugged from the electrical outlet, and then the endoscope is put in a carrying case and moved to the reprocessing room, which is separate from the procedure room and is equipped with a ventilation system to prevent toxic materials from leaking.12 As with the procedure room, the space for cleaning and disinfection is divided into a clean zone and a contaminated zone, so that contaminated endoscopes and clean endoscopes are separated. The carrying case should be large enough so as not to damage the endoscope fore-end. If the reprocessing room is far from the procedure room, the carrying case should be covered before it is moved.10
Necessary materials: cleansing solution, brush, fabric and sponge, clean water, dry fabric, ultrasonic disinfector, and compressed air.
After all the parts are disassembled, a leakage test is conducted to examine any damage in and out of the endoscope. To protect the endoscope from damage due to the cleansing solution, the test is conducted before the endoscope is soaked in the cleansing solution. If air bubbles appear, the process should be stopped and the manufacturer's guidelines should be consulted. When no bubble is observed, the endoscope is soaked in the cleansing solution as soon as possible and cleaned using a soft fabric or a sponge. The endoscope channel is brushed repeatedly until no contaminant is observed in three directions: from the suction button hole to the endoscope fore-end and the universal code, and from the biopsy channel to the endoscope fore-end.11 This process is very important because it removes 99.9% to 99.999% of the microorganisms in the endoscope.13 Then the cleansing solution is poured into the biopsy channel to drain the remaining contaminants. The parts that are separated from the endoscope are cleaned with a brush. The solid parts that are difficult to brush are soaked in the cleansing solution and cleaned using an ultrasonic disinfector. After the brushing process, the cleansing solution is completely washed out using clean water. To prevent the disinfection solution from being diluted due to the remaining water, the surface of the endoscope is wiped using a dry fabric. Compressed air is forcefully blown into all the channels to remove the remaining water.
Enzymatic detergents or neutral detergents for medical use with few bubbles are recommended as cleansing solutions.14 An ideal cleansing solution effectively permeates contaminants that contain proteins, lipids, carbohydrates and various chemical bases and separates the contaminants from the channels without damaging the endoscope.11 A cleansing solution with many bubbles is inappropriate because it reduces the contact surface with the endoscope. The cleansing solution is changed each time the endoscope is cleaned, and the enzymatic detergent is discarded after its use.
Necessary materials: disinfectant solution and automated endoscope reprocessor.
The endoscope is a semicritical item and requires a high disinfection level. There are two methods of endoscope disinfection: manual disinfection and automated endoscope reprocessing using a machine. During manual disinfection, the cleaner is exposed to an irritant and toxic disinfectant solution. Sufficient cleaning requires much manpower, and a constant level of disinfection effects is hardly obtained even though only one person performs the whole process. Therefore, automated endoscope reprocessors are frequently used these days. However, they are expensive equipment that requires regular maintenance, or they can be a source of contamination. On the other hand, if the manual disinfection method is appropriately performed, it can show the same endoscope disinfection effect as that of automated endoscope reprocessing. Therefore, appropriate disinfection method and disinfector can be selected based on the number of endoscopes to be disinfected and other factors.
The high-level disinfectants available in Korea include glutaraldehyde, ortholphthaldehyde, peracetic acid/hydrogen peroxide, and electrolyzed acid water. To achieve the effects of high-level disinfection, the manufacturer's guidelines on the disinfection environment and the exposure time to disinfectants should be followed. For example, when glutaraldehyde is used for high-level disinfection, the endoscope must be soaked at 20℃ for 20 minutes or longer.15 In addition, the biopsy channel must be filled with the disinfectant solution to achieve full-scale disinfection.7 Most high-level disinfectants are reusable, but when they are used for a long period or repeatedly, their dilution concentration decreases, and accordingly, their efficacy is reduced. Therefore, the minimum effective concentration of the endoscope disinfectant solution must be confirmed before the first disinfection is performed. If the concentration falls below the minimum level, the disinfectant must be discarded. However, even if the level is maintained, an expired solution must be discarded.16
In disinfection using an automated endoscope reprocessor, the steps are the same as those in manual disinfection: precleaning-cleaning-disinfection-rinsing-drying. Additionally, thorough hand cleaning is necessary before the endoscope is put inside the automated endoscope reprocessor. In automated disinfection, the consistent disinfection process allows more systematic management of the disinfection manpower than in manual disinfection, and when a printer is available, administrative information such as the type of disinfectant, the time and date of the disinfection, the endoscope serial number and the patient's name can be printed. In addition, the automated endoscope reprocessor has a slight water purification ability which prevents the contamination of the water supply; a heat-retaining function; and a function that monitors the disinfection process, the time of replacement of the disinfectant and changes in the disinfectant concentration. Nevertheless, a channel with an elevator is vulnerable to bacterial proliferation, so automated disinfection may not be enough. In that case, manual cleaning is required to remove the contaminants at the back or the side of the elevator using a brush or injection needles. Considering the chemical instability of the disinfectant, the remaining organic matter in the endoscope channel or the remaining water in the endoscope can reduce the concentration level of the disinfectant in the automated endoscope reprocessor, so the concentration level must be confirmed at least once a day. Poor maintenance of the automated endoscope reprocessor may make it a source of contamination, prolong the endoscopic reprocessing time and increase the disinfection cost.16-19
Factors that must be considered in selecting an automated endoscope reprocessor in the market include its price, maintenance cost, convenience of operation, and water purification function; the types of disinfectant that can be used with it; its reprocessing time, ability to print the reprocessing indicators, ultrasonic cleaning function, ventilation function for the disinfectant, and heat retaining function; the number of endoscopes that it can reprocess at the same time; and its automatic leakage test function, automatic channel-blocking monitoring function, automatic cleansing function, and mounting type.20,21
Necessary material: potable water.
This step completely removes the disinfectant that remains on the surface of the endoscope and the channel. Only potable water is used. The amount of water needed for appropriate rinsing is about three times that of the area to be rinsed.22
Necessary materials: compressed air, ethyl or isopropyl alcohol, and an endoscope cabinet.
After the endoscope is thoroughly rinsed, all its parts are disassembled. The remaining water or moisture can become a reservoir of microorganisms, so all the parts must be dried to prevent bacterial transmission and infection.7 Compressed air is blown into each channel to remove the water inside. Then the surface of the endoscope is mopped with a soft fabric, and 70% to 90% ethyl or isopropyl alcohol is poured into all its channels before compressed air is blown into them drying. Finally, the endoscope is vertically kept in a cabinet. All its detachable parts such as air/water valve, suction valve and waterproof cap are kept disassembled in storage. The cabinet is mopped with 70% alcohol daily. The suggested hang time or shelf life in some studies was 10 to 14 days after the disinfection, but no maximum hang time for the reprocessed endoscope has been determined yet.
The Association of PeriOperative Registered Nurses and the Association of Professionals in Infection Control and Epidemiology suggested a maximum hang time of 5 and 7 days, respectively.7
Go to : 
The devices used in an endoscopic examination include endoscopic components that are attached to the endoscope and accessories that are inserted into the biopsy channel during the procedure. The endoscope components are replaced for each procedure, reused after high-level disinfection and sterilized after the end of procedure. In comparison, the endoscopic accessories are divided into reusable ones such as biopsy forceps, grasping forceps, polypectomy snares, esophageal dilators, ERCP catheters, guide wires, papillotomes, needle knives, retrieval nets and balloon dilators, and disposable supplies such as injection needles, cytology brushes, and stents. The reusable accessories must be cleaned, disinfected and sterilized. Disposable supplies must not be reused. For the disinfection, the reusable components and accessories are separated from the endoscope and soaked in the cleansing solution. After the inside and outside of the pipe are mopped with a brush and a sponge, they are cleaned with an ultrasonic disinfector and rinsed with clean water. The remaining water is removed using a clean fabric and compressed air. Finally, they are kept after their sterilization or disinfection according to the manufacturer's guidelines. The air/water bottle and its connecting tube are disinfected at least daily, and sterile water should be used to fill the water bottle.
Go to : 
The use of endoscopy is increasing. Accordingly, individual and social interest in endoscopy-associated infection is also growing. While it is important to use an endoscope for the diagnosis and treatment of various diseases, it is also important to establish and observe guidelines on endoscopic disinfection to control endoscopy-associated infection.
Go to : 
References
1. Chang R. Cleaning and disinfection of gastrointestinal endoscopes. Korean J Gastrointest Endosc. 1996; 15(Suppl):877S–880S.
2. Kim YT. Introduction of proposed guideline for endsocpy disinfection. Korean J Gastrointest Endosc. 2010; 40(Suppl 1):49S.
3. Nelson DB. Infectious disease complications of GI endoscopy: part I, endogenous infections. Gastrointest Endosc. 2003; 57:546–556. PMID: 12665767.
4. Jang JY. Reprocessing of endoscopy. Korean J Gastrointest Endosc. 2011; 43(Suppl 2):170S–173S.
5. Nelson DB. Infectious disease complications of GI endoscopy: part II, exogenous infections. Gastrointest Endosc. 2003; 57:695–711. PMID: 12709700.

6. Kimmery MB, Burnett DA, Carr-Locke DL, et al. Transmission of infection by gastrointestinal endoscopy. Gastrointest Endosc. 1993; 39:885–888.

7. ASGE Quality Assurance In Endoscopy Committee. Petersen BT, Chennat J, et al. Multisociety guideline on reprocessing flexible gastrointestinal endoscopes: 2011. Gastrointest Endosc. 2011; 73:1075–1084. PMID: 21628008.

8. Lim CH, Choi MG, Kim WC, et al. Performance and cost of disposable biopsy forceps in upper gastrointestinal endoscopy: comparison with reusable biopsy forceps. Clin Endosc. 2012; 45:62–66. PMID: 22741133.

9. Kim SY, Lee HS, Hyun JJ, et al. Comparison on the efficacy of disinfectants used in automated endoscope reprocessors: PHMB-DBAC versus orthophthalaldehyde. Clin Endosc. 2011; 44:109–115. PMID: 22741121.

10. Society of Gastroenterology Nurses and Associates, Inc. Standards of infection control in reprocessing of flexible gastrointestinal endoscopes [Internet]. Chicago: Society of Gastroenterology Nurses and Associates;c2007. cited 2010 Nov 25. Available from: http://www.ascquality.org/Library/endoscopereprocessingtoolkit/SGNA%20Standards%20of%20IC%20in%20Reprocessing%20of%20Flexible%20GI%20Endoscopes.pdf.
11. Ishino Y, Ido K, Koiwai H, Sugano K. Pitfalls in endoscope reprocessing: brushing of air and water channels is mandatory for high-level disinfection. Gastrointest Endosc. 2001; 53:165–168. PMID: 11174285.

12. Alvarado CJ, Reichelderfer M. Association for Professionals in Infection Control. APIC guideline for infection prevention and control in flexible endoscopy. Am J Infect Control. 2000; 28:138–155. PMID: 10760223.

13. Rutala WA, Weber DJ. FDA labeling requirements for disinfection of endoscopes: a counterpoint. Infect Control Hosp Epidemiol. 1995; 16:231–235. PMID: 7636171.

14. Marion K, Freney J, James G, Bergeron E, Renaud FN, Costerton JW. Using an efficient biofilm detaching agent: an essential step for the improvement of endoscope reprocessing protocols. J Hosp Infect. 2006; 64:136–142. PMID: 16919846.

15. Nelson DB, Jarvis WR, Rutala WA, et al. Society for Healthcare Epidemiology of America. Multi-society guideline for reprocessing flexible gastrointestinal endoscopes. Infect Control Hosp Epidemiol. 2003; 24:532–537. PMID: 12887243.

16. Jang JY. Types, advantages and limitations of the automated endoscope washer-disinfectors. Korean J Gastrointest Endosc. 2010; 40(Suppl 1):53S–56S.
17. Ido K, Ishino Y, Ota Y, et al. Deficiencies of automatic endoscopic reprocessors: a method to achieve high-grade disinfection of endoscopes. Gastrointest Endosc. 1996; 44:583–586. PMID: 8934166.

18. Phillips G, McEwan H, McKay I, Crowe G, McBeath J. Black pigmented fungi in the water pipe-work supplying endoscope washer disinfectors. J Hosp Infect. 1998; 40:250–251. PMID: 9830597.

19. Tandon RK, Ahuja V. Non-United States guidelines for endoscope reprocessing. Gastrointest Endosc Clin N Am. 2000; 10:295–318. PMID: 10683216.

20. Muscarella LF. Advantages and limitations of automatic flexible endoscope reprocessors. Am J Infect Control. 1996; 24:304–309. PMID: 8870914.

21. American Society for Gastrointestinal Endoscopy. Petersen BT, Adler DG, et al. Automated endoscope reprocessors. Gastrointest Endosc. 2009; 69:771–776. PMID: 19327470.

22. Walter VA, DiMarino AJ Jr. American Society for Gastrointestinal Endoscopy-Society of Gastroenterology Nurses and Associates Endoscope Reprocessing Guidelines. Gastrointest Endosc Clin N Am. 2000; 10:265–273. PMID: 10683213.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download