Abstract
Background/Aims
Improvements in the endoscopic evaluation and management of gastric cancer have made it possible to determine the depth of invasion during endoscopic examination. The aim of this study was to elucidate the differences between early gastric cancer (EGC) that resembles advanced gastric cancer (AGC) and AGC that resembles EGC.
Methods
We retrieved cases of EGC-like AGC and AGC-like EGC from consecutive gastric cancers that had been completely resected. The endoscopic diagnoses and clinicopathological findings were analyzed.
Results
AGC-like EGCs were located mainly in the distal part of the stomach, whereas EGC-like AGCs were located mainly in the proximal part of the stomach (p<0.001). Sixty percent of AGC-like EGCs were moderately differentiated adenocarcinomas, while 64% of EGC-like AGCs were poorly differentiated adenocarcinomas (p=0.015). According to Lauren's classification, 68% of AGC-like EGCs were intestinal type, whereas 71% of EGC-like AGCs were diffuse type (p=0.020).
Conclusions
AGC-like EGCs predominate in the distal part of the stomach, while EGC-like AGCs predominate in the proximal part. When evaluating the depth of a gastric cancer, care should be taken not to underestimate measurements in proximal gastric cancers since they tend to be poorly-differentiated adenocarcinomas, in Lauren's diffuse type, and invade deeper than their endoscopic appearance might suggest.
Recent advances in endoscopic techniques have enabled the detection of various gastric cancers. Conventional endoscopy enables discrimination between early gastric cancer (EGC) and advanced gastric cancer (AGC), which in most cases is usually determined by histological depth of invasion.1 Identifiable characteristics include the following: 1) an elevated lesion is likely to involve mucosal invasion when its surface structure is regular and uniform without ulceration; 2) a small, slightly depressed lesion without fold convergence or bank formation frequently involves mucosal invasion; 3) a depressed lesion associated with fold convergence is likely to involve mucosal invasion when its depression is shallow and the tips of converging folds only show irregular thinning, but it is likely to involve submucosal or deeper invasion when the base of the depression is stiffened and accompanied by irregular nodules on the margin, or when the folds are elevated and enlarged; and 4) when an ulcerative lesion is surrounded by a tumorous bank, or the tips of folds are elevated and merged, it is likely to be an AGC.1 Despite all of the above characteristics, however, endoscopically diagnosed EGC is sometimes revealed to be AGC on histological examination of the resected specimen, and vice versa. Such cases are called EGC-like AGCs and AGC-like EGCs, respectively.
Regardless of lymph node (LN) metastasis, EGCs are defined as those cancers confined to the mucosal or submucosal layer, while AGCs are defined as those extending into or beyond the proper muscle layer. Some proper muscle cancers appear endoscopically as EGC, before this is confirmed by histological examination of the resected specimen.2 Notably, these EGC-like AGCs have a more favorable prognosis than Borrmann-type cancer, and have less LN metastases. In contrast, AGC-like EGCs are associated with a higher incidence of LN metastasis and predominant submucosal invasion, and therefore require extensive LN dissection.3
Since the depth of the gastric cancer invasion is strongly correlated with LN metastasis and the treatment for gastric cancers differs according to the depth of invasion,4 it is vital to discriminate between EGC and AGC during endoscopic examination. Underestimating an AGC as an EGC may lead to endoscopic resection instead of surgery, while overestimating an ECG as an AGC may lead to surgery instead of endoscopic resection. In the present study, we determined the diffe-rential clinicopathological findings of AGC-like EGCs and EGC-like AGCs, with the aim of helping to prevent such misdiagnoses.
Consecutive gastric cancer patients from August 2005 to December 2010 who underwent complete resection at Konkuk University Medical Center were included in this study. Subjects who agreed on additional analyses for microsatellite instability (MSI) and mucin phenotype were included. Patients with final diagnosis of other than adenocarcinoma were excluded from the study. This study was approved by the Institutional Review Board of Konkuk University Medical Center (KUH 1010293) which confirmed that the study was in accordance with the ethical guidelines of the Declaration of Helsinki.
Each case was diagnosed as either EGC or AGC during the endoscopic examination before the resection, and was compared with pathological results of the resected specimen (Fig. 1). A total of 14 (6.8%) cases among 207 endoscopically diagnosed EGCs were finally diagnosed as AGCs that invade the proper muscle and/or serosa. Therefore, we classified these 14 cases as EGC-like AGCs. These are AGCs with gross appearance of an EGC, but with invade deeper into the proper muscle and beyond (Fig. 2). On the other hand, 25 (14.9%) cases among 168 endoscopically diagnosed AGCs were finally diagnosed as EGCs that invade only the mucosa and/or submucosa. Therefore, we classified these 25 cases as AGC-like EGCs. These are EGCs with gross appearance of an AGC, but with invasion confined to the mucosal and/or submucosal layer (Fig. 3).
Overall, we retrieved 25 cases of AGC-like EGCs and 14 cases of EGC-like AGCs. Endoscopic findings and clinicopathological characteristics of gastric cancer such as tumor location, size, macroscopic appearance, Lauren's classification, MSI, mucin phenotype, and other pathologic findings (the depth of invasion, cell type, LN metastasis, lymphatic, venous, and perineural invasion) were analyzed.
Conventional white light endoscopy (GIF H260; Olympus, Tokyo, Japan) was used for endoscopic examination. The electronic endoscopic system consisted of EVIS-260 processor (Olympus Optical Co., Ltd., Tokyo, Japan) and the images were converted into the tagged image format using an EVIS-260 system (Olympus Optical Co., Ltd.) with a magnetic optical disk drive for each case. Electronic upper gastrointestinal endoscopic images were analyzed by one endoscopist (H.S.P.) to exclude intraobserver variability. If there were any difficulty in classification, the endoscopist referred to another endoscopist for a second opinion.
A macroscopic classification of EGC was classified as follows: type I (protruded), type IIa (superficial elevated), type IIb (flat), type IIc (superficial depressed), type III (excavated), and combination type (I+IIa, IIa+IIc, IIc+IIa, IIc+III), according to the Japanese classification of gastric carcinoma.5 In addition, advanced gastric adenocarcinoma was classified according to Borrmann classification into polypoid lesion (type I), fungating with surface ulceration (type II), large tumor mass with central ulceration (type III), and infiltrative tumor (type IV).
The cancerous lesions were cut into serial sections with the surrounding noncancerous mucosa. The size of gastric cancer was measured with the formalin-fixed specimen. Cell type, Lauren's classification (intestinal, diffuse, or mixed type), depth of invasion (lamina propria, muscularis mucosa, submucosa, proper muscle, or serosa), presence of microinvasion (lymphatic, venous, or perineural invasion) were analyzed.
MSI analysis was done as described previously in our study.6 MSI was analyzed by polymerase chain reaction (PCR) amplification with fluorescent dye-labeled primers of mononucleotide markers (BAT25 and BAT26) and dinucleotide markers (D2S123, D5S346, and D17S250) specific for the microsatellite loci. PCR was performed over 35 cycles with 1 minute at 94℃, 1 minute at 55℃, and 1 minute at 72℃ for the BAT25 and BAT26 primers. For D2S123, PCR was carried out over 35 cycles with 30 seconds at 94℃, 1 minute at 54℃, and 1 minute at 72℃. For D5S346, PCR was carried out over 36 cycles with 30 seconds at 94℃, 30 seconds at 55℃, and 30 seconds at 72℃. For D17S250, PCR was carried out over 38 cycles with 1 minute at 94℃, 1 minute at 50℃, and 1 minute at 72℃. Fluorescently labeled PCR products were detected using the ABI 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Microsatellite genotypes were categorized as a high incidence of MSI when instability was detected in 30% or more of the markers and as a low incidence of MSI when instability was detected in less than 30% of the markers.
Immunohistochemical stain was done to reveal mucin phenotypes as described previously in our study.7 Serial 4-mm sections were cut from formalin-fixed and paraffin embedded tissues and mounted on glass slides coated with silane (Matsunami, Tokyo, Japan). The immunohistochemical staining with primary antibodies, MUC5AC (45M/1, 1:2,000; Neomarker, Fremont, CA, USA), MUC 6 (MCN6.01, 1:200; Neomarker), MUC2 (996/1, 1:2,000; Neomarker), and CD10 (56C6, 1:50; Neomarker) was carried out using the iVIEW DAB detection kit (Ventana Medical Systems Inc., Tucson, AZ, USA) by the Benchmark XT (Ventana Medical Systems Inc.). Heat-induced antigen retrieval was carried out. Negative controls were carried out in all cases by omitting the primary antibodies. Hematoxylin was used for counterstaining.
The gastric cancers were subclassified into gastric and intestinal mucin phenotypes if more than 10% of cancer cells exhibited gastric (MUC5AC and/or MUC6) and intestinal (MUC2 or CD10) markers, respectively. Tumors were classified as mixed type when 10% or more of the neoplastic cells showed both gastric and intestinal markers, and classified as unclassified type when less than 10% of the neoplastic cells showed gastric and intestinal markers.
Statistical analyses were performed with commercially available statistical software, SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Differences between the AGC-like EGCs and EGC-like AGCs were analyzed using the chi-square test and Student's t-test (or Mann Whitney U-test and Kruskal Wallis test when indicated). Continuous values were expressed as mean±standard deviation (SD). A probability value of p<0.05 was considered statistically significant.
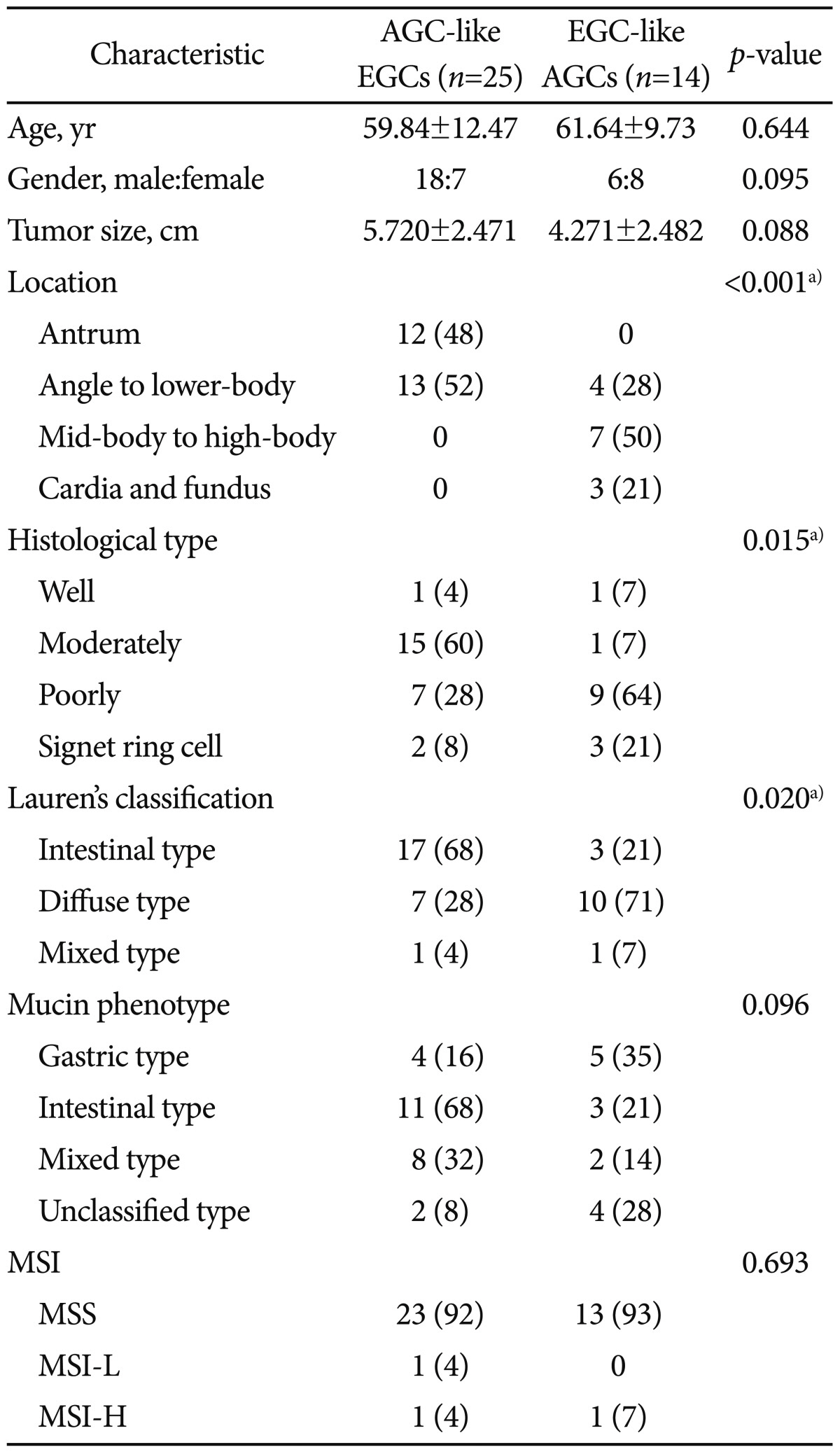
There was no difference between AGC-like EGCs and EGC-like AGCs with regard to age and gender of the subjects (Table 1). The macroscopic appearance of 25 AGC-like EGCs were consisted of three cases of Borrmann type I, three cases of Borrmann type II, 18 cases of Borrmann type III, and one case of Borrmann type IV. On the other hand, the macroscopic appearance of 14 EGC-like AGCs were consisted of two cases of elevated type, seven cases of depressive type, and five cases of combined elevated and depressed types.
When we classified the stomach into four distinct region (antrum, angle to low-body, mid-body to high-body, and cardia/fundus), AGC-like EGCs were mainly located on the antrum, angle and low-body, whereas EGC-like AGCs were mainly located on the high-body, cardia, and fundus (Fig. 4).
Cell types of AGC-like EGCs and EGC-like AGCs were significantly different (Table 1). According to the Lauren's classification, most of AGC-like EGCs were intestinal type, whereas most of EGC-like AGCs were diffuse type.
With regard to the depth of invasion, most of AGC-like EGCs (72%) invaded the submucosal layer of the stomach. Six cases (24%) invaded down to the first one-third of submucosal layer (sm1), three cases (12%) involved two-third of submucosal layer (sm2), and nine cases (36%) all three parts of submucosal layer (sm3), respectively. On the other hand, 56% of EGC-like AGCs invaded muscularis propria layer. Other four (28%) and two (14%) cases of EGC-like AGCs invaded subserosa and adjacent structure, respectively.
There were no significant differences between AGC-like EGCs and EGC-like AGCs with regard to the status of MSI and mucin phenotypes (Table 1). There were only two MSI cases among 25 AGC-like EGCs and only one MSI case among 14 EGC-like AGCs. Although it was statistically insignificant, most (68%) of the AGC-like EGCs showed higher prevalence of intestinal mucin phenotype, whereas EGC-like AGCs showed higher prevalence of gastric mucin phenotype.
This study found significant clinicopathological differences between AGC-like EGCs and EGC-like AGCs. AGC-like EGCs were usually 1) located in the distal part of the stomach, 2) showed Lauren's intestinal type, and 3) moderately differentiated adenocarcinomas, while EGC-like AGCs were usually 1) located in the proximal part of the stomach, 2) showed Lauren's diffuse type, and 3) poorly differentiated adenocarcinomas. These findings might be attributable to anatomical variations inside the stomach, in which the wall thickness differs according to the location. It is reported that the stomach wall is thinner in the upper than in the lower part of the stomach: the mean antral thicknesses of the anterior and posterior walls were found to be 5.0±1.9 and 5.2±1.7 mm (mean±SD), respectively, and 5.1±1.6 mm overall, whereas the gastric body was found to be thinner (2.0±0.4 mm) than the gastric antrum.8 Therefore, we can assume that tumor cells can invade more easily and deeply (i.e., into the proper muscle and beyond) in the upper part of the stomach than in the thicker, lower part. Care should therefore be taken during the diagnostic process, since shallow-looking AGCs (so called EGC-like AGCs) in the upper part of the stomach might lead to undertreatment, such as endoscopic resection.
Our findings on the location of the lesions are consistent with those of previous studies.1,2,9 Interestingly, for the EGC-like AGC, the rate of LN metastasis was low compared with Borrmann-type cancer, and a curative resection was performed in 97.7% of the patients.2 In the latter study, 38% of proper muscle-layer cancers were diagnosed as AGC-like EGC on macroscopic examination. Patients with these cancers were younger and the tumor occurred in the upper and middle regions, factors differing from the situation in patients with Borrmann type cancer. Another study revealed that when the tumor was an elevated type or when it was located in the antrum, the endoscopically estimated depth of tumor invasion tended to be greater than the true depth.1 It has also been reported that the accuracy of endoscopic staging tends to be lower for lesions located in the upper third of the stomach, with a flat and depressed configuration, at least 1.0 cm in size, an undifferentiated histology, or submucosal invasion.9
It was possible to distinguish between AGC-like EGCs and EGC-like AGCs based on histological cell types in our study. Lauren's diffuse type and poorly differentiated adenocarcinomas were associated more with EGC-like AGCs than with AGC-like EGCs, in line with the finding that these cancers frequently exhibit an extensive and high degree of penetration. This is consistent with our previous study that intestinal-type gastric cancers are more often antral cancers that are related to gastric adenoma, whereas diffuse-type gastric cancers are not.10 Therefore, EGC-like AGCs tend to be more invasive - deeper invasion or more LN metastasis - in their biological behavior.
The main limitation of our study is that we did not apply endoscopic ultrasonography (EUS) or new imaging techniques such as narrow band imaging or virtual gastroscopy.11-13 In a previous study comparing EUS and conventional endoscopy for staging depth of invasion in early gastric cancer, EUS was found to reduce misdiagnosis.11 In the gastric body-cardia, conventional endoscopy showed a 23.9% of understaging rate due to its forward-viewing feature. The authors explained that it is difficult to achieve frontal view of the lesion with a forward-viewing instrument, while it can be easily examined by EUS because of the laterally-directed ultrasound beam. Therefore, the combination of conventional endoscopy and EUS could be helpful for correct evaluating depth of invasion, because EUS can compensate for understaging of lesions by conventional endoscopy, especially in the upper part of the stomach. Despite this limitation, our use of a 5-year data-collection period revealed that AGC-like EGCs are more frequently noticed than EGC-like AGCs because of the higher incidence of gastric cancers in the distal part of the stomach than in the proximal part. In addition, the accuracy of endoscopic diagnosis seems to decrease when the depth of invasion is greater in EGC.14 This indicates that typical mucosal cancers can be diagnosed easily as EGCs, but submucosal cancers with further morphological changes in the folds sometimes obscure the depth of invasion, reducing the accuracy of this diagnostic method.
In conclusion, AGC-like EGCs and EGC-like AGCs exhibit different clinicopathological characteristics. We found that the distal part of the stomach is commonly involved in AGC-like EGCs, whereas the proximal part of the stomach is commonly involved in EGC-like AGCs. Therefore, care should be taken not to underestimate small AGCs in the high-body, cardia, or fundus as EGCs. Similarly, care should be taken not to overestimate large EGCs in the antrum, angle, and low-body of the stomach as AGCs. Together, these findings suggest that when evaluating the depth of a gastric cancer using conventional endoscopy, great care should be taken not to overestimate distal gastric cancers and not to underestimate proximal gastric cancers, since the latter tend to be revealed as poorly differentiated, Lauren's diffuse-type cancers, and thus invade deeper than indicated endoscopically.
References
1. Sano T, Okuyama Y, Kobori O, Shimizu T, Morioka Y. Early gastric cancer. Endoscopic diagnosis of depth of invasion. Dig Dis Sci. 1990; 35:1340–1344. PMID: 2226095.
2. Maehara Y, Anai H, Moriguchi S, Watanabe A, Tsujitani S, Sugimachi K. Gastric carcinoma invading muscularis propria and macroscopic appearance. Eur J Surg Oncol. 1992; 18:131–134. PMID: 1582506.
3. Kitamura K, Yamaguchi T, Nishida S, et al. Early gastric cancer mimicking advanced gastric cancer. Br J Cancer. 1997; 75:1769–1773. PMID: 9192979.

4. Coda S, Lee SY, Gotoda T. Endoscopic mucosal resection and endoscopic submucosal dissection as treatments for early gastrointestinal cancers in Western countries. Gut Liver. 2007; 1:12–21. PMID: 20485653.

5. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma: 2nd English Edition. Gastric Cancer. 1998; 1:10–24. PMID: 11957040.
6. Choe WH, Lee SY, Lee JH, et al. High frequency of microsatellite instability in intestinal-type gastric cancer in Korean patients. Korean J Intern Med. 2005; 20:116–122. PMID: 16134765.

7. Han HS, Lee SY, Lee KY, et al. Unclassified mucin phenotype of gastric adenocarcinoma exhibits the highest invasiveness. J Gastroenterol Hepatol. 2009; 24:658–666. PMID: 19175827.

8. Pickhardt PJ, Asher DB. Wall thickening of the gastric antrum as a normal finding: multidetector CT with cadaveric comparison. AJR Am J Roentgenol. 2003; 181:973–979. PMID: 14500212.
9. Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc. 2011; 73:917–927. PMID: 21316050.

10. Lee SY, Han HS, Lee KY, et al. Sonic hedgehog expression in gastric cancer and gastric adenoma. Oncol Rep. 2007; 17:1051–1055. PMID: 17390043.

11. Yanai H, Matsumoto Y, Harada T, et al. Endoscopic ultrasonography and endoscopy for staging depth of invasion in early gastric cancer: a pilot study. Gastrointest Endosc. 1997; 46:212–216. PMID: 9378206.

12. Akashi K, Yanai H, Nishikawa J, et al. Ulcerous change decreases the accuracy of endoscopic ultrasonography diagnosis for the invasive depth of early gastric cancer. Int J Gastrointest Cancer. 2006; 37:133–138. PMID: 18080789.

13. Furukawa K, Miyahara R, Itoh A, et al. Diagnosis of the invasion depth of gastric cancer using MDCT with virtual gastroscopy: comparison with staging with endoscopic ultrasound. AJR Am J Roentgenol. 2011; 197:867–875. PMID: 21940574.

14. Yin JX, Oda I, Suzuki H, Gotoda T, Shimoda T, Saito D. Endoscopic diagnosis of gastric cancer invasion depth. Nihon Shokakibyo Gakkai Zasshi. 2009; 106:1603–1609. PMID: 19893290.
Fig. 1
Study flow of our study. Of consecutive gastric cancers that were resected and analyzed for microsatellite instability and mucin phenotype, we retrieved 25 advanced gastric cancer (AGC)-like early gastric cancers (EGCs) and 14 EGC-like AGCs.

Fig. 2
An advanced gastric cancer-like early gastric cancer in a 48-year-old man. (A) Endoscopic image shows a huge mass extending from prepyloric antrum to lower-body. Macroscopic appearance shows an irregularly elevated lesion, measuring 8.6×7.1 cm. (B) Histological examination demonstrates submucosal cancer invading down to the deepest submucosal layer (H&E stain, ×12.5).

Fig. 3
An early gastric cancer-like advanced gastric cancer in a 69-year-old man. (A) Endoscopic image shows a depressed lesion with irregular margin in the cardia. Macroscopic appearance shows a slightly depressed lesion measuring 2.5×2.5 cm. (B) Histological examination demonstrates invasion confined to the muscularis propria layer (H&E stain, ×12.5).

Fig. 4
Locations of advanced gastric cancer (AGC)-like early gastric cancers (EGCs) and EGC-like AGCs. AGC-like EGCs are mostly located in the distal part of the stomach, whereas EGC-like AGCs are mostly located in the proximal part of the stomach (p<0.001).





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download