Abstract
As a rare complication of percutaneous endoscopic gastroscopy (PEG), a gastrocolocutaneous fistula may occur after PEG placement. This paper reports an interesting case which PEG tube unintentionally penetrated transverse colon during PEG. A 72-year-old female patient who suffered from medullary infarction underwent PEG procedure for enteral nutrition, and fecal materials were observed 6 days after the procedure. Transverse colon located in antero-superior site of stomach was observed through abdominal computed tomography, and also the wrong inserted tube was found through gastroscopy and colonoscopy. Endoscopic treatment for the fistula was performed by the use of hemo-clip and detachable snare, closure of the fistula was finally confirmed 6 days after the endoscopic procedure. Therefore, the gastrocolocutaneous fistula should be considered as one of the complications of PEG when fecal material is observed through PEG tube in a few days after PEG procedure and endoscopic treatment can be feasible in this case.
In terms of enteral nutritional support, percutaneous endoscopic gastroscopy (PEG) is widely accepted as a safe and effective approach. However, some general complications have been reported, such as skin infection of peri-gastrostomy site, ileus, leakage, tubal obstruction, stomach perforation, hemorrhage, peritonitis, pulmonary aspiration and buried bumper syndrome etc.1-6 Gstrocolocutaneous fistula, defined as an epithelial linkage between the mucosa of the stomach, colon and skin, is rare but also is included as one of the complications. The typical symptoms of gastrocolocutaneous fistula are severe diarrhea, malnutritional status, gram negative pulmonary infection and feculent vomiting,7 and the fistula is diagnosed by complementary work-up of fistulography using barium or gastrografin injection through the PEG tube, abdominal computed tomography (CT), gastroscopy and colonoscopy. This report will present the case of a patient who had a gastrocolocutaneous fistula after PEG procedure and treated by endoscopic procedure.
A PEG tube insertion was performed to a 72-year-old female patient who was suffering from medullary infarction for the purpose of enteral nutrition (Fig. 1A). There were no major symptoms observed for 2 days after PEG insertion. Three days after the PEG, however, yellowish fecal materials were drained through the PEG tube during gastric remnant volume examination before feeding. Six days after the procedure, we performed the endoscopy, and the fecal materials were attached to gastric wall and the end of the PEG tube was away from the location where it had been originally positioned, and was partially buried (Fig. 1B). The tube was immediately removed, and peripheral total parenteral nutrition without oral intake was started. At the follow-up the day after removing the PEG tube, abdominal CT was conducted, and colonoscopy was also conducted 3 days after the abdomen CT since there was no spontaneous closure of gastrostomy site and fecal drainage was sustained. On the abdomen CT, the transverse colon was located in antero-superior site of the stomach (Fig. 2) and the fistula of the middle of transverse colon was found during colonoscopy. However, the gastrocolocutaneous fistula was not shown during fistulography which was conducted after the injection of contrast media through nasogastric tube. The leakage was observed continuously after removing the PEG tube. Endoscopic suture using hemo-clips was carried out during colonoscopy (Fig. 3A, B).
During gastroscopic examination the day after the colonoscopy, some of the end of hemo-clip located at the transverse colon was shown from gastric cavity side. This confirmed that there was a fistula between stomach and transverse colon. This fistula at the gastric side was also sutured with hemo-clips, and then a detachable snare (MAJ-254; Olympus, Tokyo, Japan) was applied for concrete suture (Fig. 3C, D). The closure of the fistula was confirmed at the gastroscopy conducted 6 days after the endoscopic procedure (Fig. 4).
PEG was suggested in the early eighties as a new approach to substitute operative methods in the equipment of a gastrostomy.8 The majority of PEG procedures are applied for neurological disorders such as stroke or traumatic irreversible brain damage, but it can be also applied in cases of anatomical problems such as a correction of cleft lip and palate, and in cases of head and neck injury caused by radiation therapy.
PEG requires minimal sedation, not general anesthesia, and can be performed within 15 to 30 minutes with 95% success rates,9 but approximately 17% out of total cases shows relevant complications according to a previous research.10 Specifically, gastrocolocutaneous fistula caused by PEG procedure was also reported in 2% to 3% of the total incidences.11 In order to prevent the gastrocolocutaneous fistula, Croaker et al.12 suggested that excessive inflation of air into the stomach should be refrained during PEG procedure since too much air in the stomach makes the greater curvature of the stomach rotate forward and makes the gastrocolic omentum and the transverse colon, originally located inferior to the stomach, move anterior to the stomach. In this condition, PEG tube can be placed into the stomach, penetrating the transverse colon. According to Patwardhan et al.,13 among 12 cases of gastrocolocutaneous fistula after PEG insertion, all of the cases showed PEG entrance site located in the posterior wall of the stomach. In our cases, however, PEG entrance site was the at the greater curvature of the stomach.
When the gastrocolocutaneous fistula occurs after PEG procedure, general symptoms such as diarrhea can be observed immediately. However, symptoms like fecal drainage through the PEG tube can be appeared after several days of asymptomatic period as described in our case study. There are insufficient data on the duration for the PEG tube removal site to be collapsed and closed. Also, clinically, it is not quite certain how long is needed for the closure of the removal site when removing the tube due to inappropriate PEG function. It is assumed that the spontaneous closure of fistula occurs probably within a couple of days, but the spontaneous closure can be disturbed by delayed gastric emptying, slow recovery of the wounded site, and the leakage of gastric juice through fistula.14 Also, there is not enough data in the literature to propose standard therapeutic approaches to PEG malposition associated with gastrocolocutaneous fistula. The decisions on therapeutic timing and methods are based on the experience and discretion of the endoscopist. In our case, the fistula, which was not spontaneously closed 5 days after the PEG tube removal, was first sutured with hemo-clips at the transverse colon side, then sutured the next day with hemo-clips at the gastric side and ligated with a detachable snare.
With an increase of the elderly, the incidence rate of neurologic diseases such as stroke, brain hemorrhage, and cases of central nerve injury by trauma due to traffic accident is also increasing. Accordingly, the need for enteral feeding by PEG is on the rise and the cases of complications associated with PEG are expected to increase. The progression to a fatal situation can be minimized by early detection of these complications. If fecal materials are drained in the evaluation of remnant stomach volume after a few days in patients undergoing PEG, the possibility of gastrocolocutaneous fistula, though rare, should be kept in mind. It is meaningful that endoscopic treatment can be feasible if the complications were detected early by examinations such as the upper and lower gastrointestinal endoscopy, abdominal CT or fistulography.
References
1. Larson DE, Burton DD, Schroeder KW, DiMagno EP. Percutaneous endoscopic gastrostomy. Indications, success, complications, and mortality in 314 consecutive patients. Gastroenterology. 1987; 93:48–52. PMID: 3108063.
2. Mamel JJ. Percutaneous endoscopic gastrostomy. Am J Gastroenterol. 1989; 84:703–710. PMID: 2500845.

3. Wicks C, Gimson A, Vlavianos P, et al. Assessment of the percutaneous endoscopic gastrostomy feeding tube as part of an integrated approach to enteral feeding. Gut. 1992; 33:613–616. PMID: 1612476.

4. Hull MA, Rawlings J, Murray FE, et al. Audit of outcome of long-term enteral nutrition by percutaneous endoscopic gastrostomy. Lancet. 1993; 341:869–872. PMID: 8096573.

5. Onishi J, Kuzuya M, Sakaguchi H. Survival rate after percutaneous endoscopic gastrostomy in a long-term care hospital. Clin Nutr. 2004; 23:1248–1249. PMID: 15380920.

6. Schrag SP, Sharma R, Jaik NP, et al. Complications related to percutaneous endoscopic gastrostomy (PEG) tubes. A comprehensive clinical review. J Gastrointestin Liver Dis. 2007; 16:407–418. PMID: 18193123.
7. Kierstead BS, Khan A, Ruppert E, Bobo R. Colocutaneous gastric fistula: a complication of percutaneous endoscopic gastrostomy. Pediatr Surg Int. 1991; 6:134–135.

8. Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980; 15:872–875. PMID: 6780678.

9. Huang SY, Levine MS, Raper SE. Gastrocolic fistula with migration of feeding tube into transverse colon as a complication of percutaneous endoscopic gastrostomy. AJR Am J Roentgenol. 2005; 184(3 Suppl):S65–S66. PMID: 15728025.

10. Smyth GP, McGreal GT, McDermott EW. Delayed presentation of a gastric colocutaneous fistula after percutaneous endoscopic gastrostomy. Nutrition. 2003; 19:905–906. PMID: 14559330.

11. Khattak IU, Kimber C, Kiely EM, Spitz L. Percutaneous endoscopic gastrostomy in paediatric practice: complications and outcome. J Pediatr Surg. 1998; 33:67–72. PMID: 9473103.

12. Croaker GD, Najmaldin AS. Laparoscopically assisted percutaneous endoscopic gastrostomy. Pediatr Surg Int. 1997; 12:130–131.

13. Patwardhan N, McHugh K, Drake D, Spitz L. Gastroenteric fistula complicating percutaneous endoscopic gastrostomy. J Pediatr Surg. 2004; 39:561–564. PMID: 15065028.

14. Shellito PC, Malt RA. Tube gastrostomy. Techniques and complications. Ann Surg. 1985; 201:180–185. PMID: 3918515.
Fig. 1
Gastroscopic findings. (A) Percutaneous endoscopic gastroscopy (PEG) tube was inserted. (B) Six days after PEG, it was observed that the fecal materials were attached to gastric wall and that the end of PEG tube was partially buried.
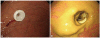
Fig. 2
Contrast enhanced computed tomography scan findings, 1 day after percutaneous endoscopic gastroscopy tube removal. Red arrow indicates transverse colon located in anterosuperior site of stomach. A fistular tract between stomach, transverse colon and abdominal wall was also observed.

Fig. 3
Endoscopic findings. (A) A part of fistula was shown at colonoscopic finding. (B) During colonoscopy, primary closure was performed with hemo-clips. (C) Some of the end of hemo-clip located at the transverse colon was shown from gastric cavity side at gastroscopic finding. (D) During gastroscopy, the fistula at gastric side was also sutured with hemo-clips and then detachable snare was applied for concrete suture.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download