Abstract
Background/Aims
Rectal carcinoid tumors, at diagnosis, are as small as 10 mm or less in about 80% of patients. These tumors are generally removed by endoscopic resection. The aim of this study was to compare treatment efficacy and safety between endoscopic submucosal resection with band ligation (ESMR-L) and conventional polypectomy.
Methods
Between January 2005 and September 2010, a total of 88 patients, who visited at Busan Paik Hospital and Kosin University Gospel Hospital for endoscopic resection of rectal carcinoid, were reviewed, retrospectively.
Results
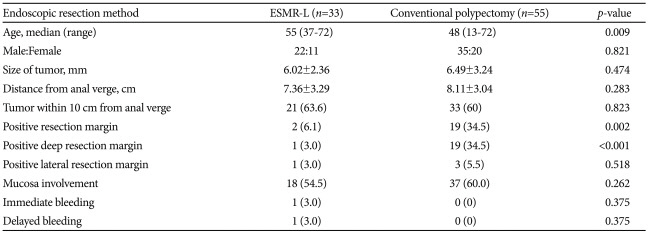
Thirty-three cases were treated by ESMR-L, and 55 cases by conventional polypectomy. There were no significant difference in the size of tumor between ESMR-L group and polypectomy group (6.02±2.36 vs. 6.49±3.24 mm, p=0.474). The rate of positive resection margin was significantly lower in ESMR-L group (2/33, 6.1%) than in polypectomy group (19/55, 34.5%; p=0.002). The rate of positive vertical resection margin, among others, was markedly lower in ESMR-L group (1/33, 3.0%) compared to polypectomy group (19/55, 34.5%; p<0.001).
Carcinoid tumor, also called neuroendocrine tumor, has a potential of progressing to malignancy. In 1907, Oberndorfer1 first described 'karzinoide' for tumors with the appearance of clinically benign course but pathologically closer to malignancy, and then Gosset and Masson2 first established the concept of endocrine tumor in 1914.
Carcinoid tumors may affect various organs with neuroendocrine cells, such as gastrointestinal (GI) tract, bronchus, lung, thymus, kidney, ovary and testis, but 75% to 90% of carcinoid tumors occur at the GI tract.3-5 The incidence of GI carcinoid tumor is around 2.5 to 5 per 100,000 people, but the incidence and prevalence is increasing because of the recent technical improvement in endoscopy and radiology.6 Rectum is the third commonly affected lesion of GI carcinoid tumor, following small intestine and colon including appendix,7 and the prevalence is reported around 0.05% to 0.07% with endoscopic screening.8
Rectal carcinoid is found asymptomatic in about 50% of patients, who are diagnosed early as a small-size asymptomaticcarcinoid tumor incidentally by colonoscopy, sigmoidoscopy or rectoscopy performed for regular check-up. About 80% of typical rectal carcinoid cases are 10 mm or less in size, restricted in submucosal layer without metastasis, its 5-year survival rate estimated around 88.3%,7 which is why most rectal carcinoid cases, with sizes of 10 mm or less, are removed by minimal invasive procedures such as endoscopic or transanal resection.9 However, more than 75% of rectal carcinoids are infiltrat-ed into the submucosal layer, making complete resection with polypectomy more difficult,10 and requiring additional surgical interventions in case of incomplete resection. Various endosco-pic resection methods have been introduced, therefore, to obtain sufficient resection margin including submucosal lesion.7
Endoscopic submucosal resection with band ligation (ESMR-L) ligates tumor with a band and places a snare below the band for resection, enabling deep vertical resection margin and higher complete resection rate.11,12 We compared the outcomes of treatment between ESMR-L and conventional snare polypectomy, among endoscopic resection methods for 10 mm or less rectal carcinoid without metastasis.
The study was performed in rectal carcinoid cases who received endoscopic resection at Busan Paik Hospital and Kosin University Gospel Hospital between January 2005 and September 2010. Rectal carcinoids 10 mm or less in size on endoscopy and without metastasis on imaging such as abdominal computed tomography (CT) were defined as the indication of endoscopic resection. We retrospectively investigated the patients' ages, genders, tumor sizes, locations and malignancy potential at diagnosis, as well as the histologic findings, tumor sizes, involvement of the resected margins and mucosal and proper muscle invasion of the resected tumors.
Tumor was first elevated by submucosal injection of the mixed solution (injection fluid) of hypertonic saline, indigo-carmine and epinephrine diluted to 1:1,000, and then the lesion was snared and resected by using electrocautery (Fig. 1).
The lesion was elevated by submucosal injection of the same injection fluid, and then the distal end of the endoscope was equipped with the band ligator (Akita Sumitomo Bakelite Co., Ltd., Tokyo, Japan). The tumor was ligated with the band ligator while suctioning, which was then resected by using electrocautery with the snare placed below the band (Fig. 2).
Student t-test was performed for analysis of patients' age, tumor sizes and distances from anal verge of each group. Gender, ratio of positive resection margin (both vertical and lateral), mucosal involvement and complication were analyzed using Fisher's exact test; variables affecting complete resection rate were validated with multivariate logistic regression method. A p-value of less than 0.05 was considered statistically significant. SPSS version 18.0 for windows (SPSS Inc., Chicago, IL, USA) was used for every statistical analysis.
A total of 88 patients, who received endoscopic resection for rectal carcinoid, were included in the study. Patients' age ranged from 13 to 72 years; 57 were male and 31 were female patients. Tumor size ranged from 2 to 10 mm, and the distance from anal verge was 2 to 15 cm. Thirty-three out of the overall patients received ESMR-L.
The rate of positive resection margin was significantly higher in the polypectomy group (19/55 [34.5%]) compared to the ESMR-L group (2/33 [6.1%], p=0.002), with marked difference in vertical resection margin (19/55 [34.5%] and 1/33 [3.0%], respectively, p<0.001). Patients in the ESMR-L group were older than those in the polypectomy group, which did not affect the study result. Immediate and delayed post-resection bleeding was found, 1 case for each, in the ESMR-L group, which was not statistically significant. There was no case of perforation in either group (Table 1).
Pathologically complete resection was defined by the existence of normal tissues in the mucosa or submucosal layer surrounding both vertical and lateral aspects of the tumor without any tumor cell in the resection margin on microscopy. Complete resection rate was significantly higher when the tumor was nearer from the anal verge (p=0.003) and in the ESMR-L group (p=0.002) (Table 2).
Variables that could affect the complete resection rate, such as age and distance from the anal verge, were analyzed and were found significant only in the ESMR-L group with the odds ratio of 0.095 (95% confidence interval, 0.018-0.505; p=0.006) (Table 3).
GI carcinoid tumor is a condition with the incidence of 2.5 to 5 per 100,000 people, and the incidence is ever increasing because of the technical improvement in endoscopy and radiology. Modlin et al.6 reported that the incidence have increased 4.6 times for small intestine and 7.2 times for the entire GI tract, over the past 3 decades. Rectal carcinoid is a rare condition accounting for only 1.1% to 1.3% of the overall rectal tumor cases.13,14 Nonetheless, rectum is the third common lesion of GI carcinoid tumor,7 accounting for 36% to 72.3% in South Korea, which is higher than 10% to 15% reported in Western countries.15-19
The two most significant prognostic factors of carcinoid tumor are the tumor size and microinvasion to the proper muscle layer. In large studies, the rate of lymph node metastasis of rectal carcinoid was found around 3% to 9.8% when the size was 10 mm or less, 17% to 81% when 10.1 to 20 mm, and 60% to 80% when 20 mm or more.6,7,20-26 About 80% of typical rectal carcinoid cases are 10 mm or less in size, restricted in submucosal layer with less metastasis, contributing to its favorable prognosis with 5-year survival rate estimated around 88.3%.7 For this reason, local treatment with endoscopic resection or minimal invasive surgery, rather than radical resection, is preferred in many cases.8,9,22,25-28 Rectal carcinoid of 10 mm or less in size, without lymphovascular, proper muscle invasion or lymph node metastasis, is reported to have good prognosis of around 98.9% to 100% of 5-year survival rate.6,21,22,29
Kobayashi et al.30 reported that tumors of 10 mm or less in size, without invasion to the proper muscle layer on endoscopic ultrasound nor concomitance with tumor retraction or ulcer, are completely resectable with polypectomy. It is considered diffi-cult, however, to achieve pathologically complete resection with polypectomy, since 75% of rectal carcinoids are infiltrated into the submucosal layer.10 The complete resection rate of polypectomy varies from 28.6% to 100%, according to the literature; it is commonly known as 64.8% on average,8,11,31-37 which is comparable to the mean 65.5% in this study. Incomplete resection might cause additional endoscopic or surgical therapy to remove remnant tumor, which is why we need a specific therapeutic endoscopic procedure that could provide deeper, wider resection for removal of submucosal lesion with sufficient resection margin.
Rectum is fixed to the retroperitoneum, facilitating endoscopy, and peritonitis is rarely occurred even when the serosa was perforated; therefore, options of therapeutic endoscopic methods are wider for rectum compared to other organs, including strip biopsy,31,32 suction polypectomy,34,35 ESMR-L38,39 and endoscopic submucosal dissection35-37 as the most effective endoscopic treatment methods reported for rectal carcinoid.
ESMR-L provides deeper vertical resection margin, hence higher complete resection rate, by suction and band ligation of the lesion followed by resection with snare placed below the ligated band.11,12 Complete resection rate of ESMR-L was reported around 95.2% to 100% in the literature11,12,38,39 and 93.9% in this study, which are exceptionally higher than that of conventional polypectomy. Studies by Berkelhammer et al.38 and Moon et al.39 reported 100% of complete resection rates; but with only 5 and 11 subjects included, respectively, those results were not suitable for generalization. The study by Mashimo et al.12 was performed in a larger population of 63 patients, but was limited due to the lack of comparison with polypectomy as well as the data on whether the positive resection margins occurred in 3 patients were vertical or lateral. The study by Ono et al.11 is the only one that performed comparison with polypectomy, with more than 10 cases per each group. The present study is distinct from the previous studies in including as many cases as 33 patients and 55 patients per each group, with more patients in the control (polypectomy) group. This study also performed analysis on both lateral and vertical resection margins, which could provide, through follow-up, a basis for the analysis on the risk of recurrence in the future.
Theoretically, ESMR-L is expected to have higher risk of perforation and significant bleeding than polypectomy due to the vertically deeper resection. Five patients (7.9%) in the study by Mashimo et al.12 also experienced bleeding requiring endoscopic coagulation procedure. Endoscopic submucosal resection with double ligation technique is reported to be able to achieve deep resection margin with reduced complications at the same time, by placing a detachable snare below the ligated band and resecting in the between of them.39 We did not use a detachable snare in this study, but not a case of perforation occurred, and the one case of each (6.1%) immediate and delayed bleeding were mild cases that were controllable with endoscopy without a need for surgery for bleeding control or blood transfusion, suggesting that ESMR-L was generally and relatively safe.
Ishikawa et al.40 noted that the lower rectum has significantly thicker internal wall than the upper rectum or colon. The wall thickness of the upper rectum is not significantly greater than that of the sigmoid colon. The internal wall of the lower rectum is supported by the surrounding connective tissues, thus it is not perforated by sufficient suction for endoscopic resection and enables deeper vertical resection margin. That seems to explain why the ESMR-L was superior in complete resection rate than conventional polypectomy. The findings of this study also suggests that lesions, located in the within 10 cm of lower rectum, achieved better complete resection rate by endoscopic treatment.
Cases with positive resection margin, after the endoscopic resection of carcinoid tumor, were followed without additional treatment, and have been reported not to have a remnant tumor, relapse or metastasis. The heat generated during the resection might have destroyed the neighboring remnant tumor cells,8,31,33,40,41 but we should not exclude the possibility that recurrence or metastasis was not detected in short-term follow-up due to the slow progression of carcinoid tumor.19
This study has several limitations. First, the fact that each of the two institutions of this study preferred different endoscopic resection methods might have been a confounding factor. The results of this study on complete resection rate were similar to previous reports, though, suggesting the collected data was appropriate. Second, as the nature of a retrospective study, we could not exclude the possibility of selection bias. The possibility seems low, however, because the difference of tumor size and location between the two groups was not statistically significant, although some of the variables were different for each group. The results and period of the follow-up, after the endoscopic resection, were not specified. Prospective, multi-center studies, with validated protocol and longer follow-up period, are warranted.
In conclusion, Small rectal carcinoids, without metastasis, are generally treated with endoscopic resection. A specific endoscopic resection method is required, however, for conventional polypectomy is more likely to cause incomplete resection. ESMR-L, with deeper resection and easier procedure, is known to be effective for small rectal carcinoids. This study also suggested that ESMR-L achieved higher complete resection rate than conventional polypectomy, in treating rectal carcinoids of 10 mm or less in size. Further prospective multi-center studies, with longer follow-up period, are required on this issue.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (20100005190).
References
1. Oberndorfer S. Karzinoide Tumoren des Dünndarms. Frankf Z Pathol. 1907; 1:426–429.
2. Gosset A, Masson P. Tumeurs endocrines de l'appendice. Presse Med. 1914; 22:237–240.
3. Marshall JB, Bodnarchuk G. Carcinoid tumors of the gut. Our experience over three decades and review of the literature. J Clin Gastroenterol. 1993; 16:123–129. PMID: 8463615.
4. Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997; 79:813–829. PMID: 9024720.

6. Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008; 9:61–72. PMID: 18177818.

7. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003; 97:934–959. PMID: 12569593.

8. Matsui K, Iwase T, Kitagawa M. Small, polypoid-appearing carcinoid tumors of the rectum: clinicopathologic study of 16 cases and effectiveness of endoscopic treatment. Am J Gastroenterol. 1993; 88:1949–1953. PMID: 8237948.
9. Okamoto Y, Fujii M, Tateiwa S, et al. Treatment of multiple rectal carcinoids by endoscopic mucosal resection using a device for esophageal variceal ligation. Endoscopy. 2004; 36:469–470. PMID: 15100972.

10. Matsumoto T, Iida M, Suekane H, Tominaga M, Yao T, Fujishima M. Endoscopic ultrasonography in rectal carcinoid tumors: contribution to selection of therapy. Gastrointest Endosc. 1991; 37:539–542. PMID: 1936832.

11. Ono A, Fujii T, Saito Y, et al. Endoscopic submucosal resection of rectal carcinoid tumors with a ligation device. Gastrointest Endosc. 2003; 57:583–587. PMID: 12665777.

12. Mashimo Y, Matsuda T, Uraoka T, et al. Endoscopic submucosal resection with a ligation device is an effective and safe treatment for carcinoid tumors in the lower rectum. J Gastroenterol Hepatol. 2008; 23:218–221. PMID: 18289355.

13. Godwin JD 2nd. Carcinoid tumors. An analysis of 2,837 cases. Cancer. 1975; 36:560–569. PMID: 1157019.
14. Teleky B, Herbst F, Langle F, Neuhold N, Niederle B. The prognosis of rectal carcinoid tumours. Int J Colorectal Dis. 1992; 7:11–14. PMID: 1588217.

15. Jung KC, Kim HS, Song SY, Choe GY, Kim YI. Carcinoid tumors of the gastrointestinal tract: analysis of 36 cases. Korean J Pathol. 1996; 30:396–407.
16. Jang NS, Sung KC, Pyeon YJ, et al. Clinical reviews of patients with carcinoid tumor. Korean J Gastroenterol. 1997; 30:179–186.
17. Chung MG, Kang DH, Kim ES, et al. Clinical study of gastrointestinal carcinoid tumors. Korean J Gastrointest Endosc. 2002; 24:135–142.
18. Chang JH, Kim SW, Chung WC, et al. Clinical review of gastrointestinal carcinoid tumor and analysis of the factors predicting metastasis. Korean J Gastroenterol. 2007; 50:19–25. PMID: 18172355.
19. Lee MH, Shin SJ, Jeon SJ, et al. Clinical characteristics of gastrointestinal carcinoid tumors. Korean J Gastrointest Endosc. 2010; 40:347–351.
20. Jetmore AB, Ray JE, Gathright JB Jr, McMullen KM, Hicks TC, Timmcke AE. Rectal carcinoids: the most frequent carcinoid tumor. Dis Colon Rectum. 1992; 35:717–725. PMID: 1643994.
21. Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005; 103:1587–1595. PMID: 15742328.
22. Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, Nagawa H. Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut. 2007; 56:863–868. PMID: 17213340.

23. Sauven P, Ridge JA, Quan SH, Sigurdson ER. Anorectal carcinoid tumors. Is aggressive surgery warranted? Ann Surg. 1990; 211:67–71. PMID: 2294847.
24. Tsukamoto S, Fujita S, Yamaguchi T, et al. Clinicopathological characteristics and prognosis of rectal well-differentiated neuroendocrine tumors. Int J Colorectal Dis. 2008; 23:1109–1113. PMID: 18594844.

25. Kwaan MR, Goldberg JE, Bleday R. Rectal carcinoid tumors: review of results after endoscopic and surgical therapy. Arch Surg. 2008; 143:471–475. PMID: 18490556.
26. Ramage JK, Goretzki PE, Manfredi R, et al. Consensus guidelines for the management of patients with digestive neuroendocrine tumours: well-differentiated colon and rectum tumour/carcinoma. Neuroendocrinology. 2008; 87:31–39. PMID: 18097130.

27. Scherübl H. Options for gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008; 9:203. PMID: 18308249.

28. Shinohara T, Hotta K, Oyama T. Rectal carcinoid tumor, 6 mm in diameter, with lymph node metastases. Endoscopy. 2008; 40(Suppl 2):E40–E41. PMID: 18302079.
29. Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy. 2009; 41:162–165. PMID: 19214898.

30. Kobayashi K, Katsumata T, Yoshizawa S, et al. Indications of endoscopic polypectomy for rectal carcinoid tumors and clinical usefulness of endoscopic ultrasonography. Dis Colon Rectum. 2005; 48:285–291. PMID: 15714250.

31. Iishi H, Tatsuta M, Yano H, Narahara H, Iseki K, Ishiguro S. More effective endoscopic resection with a two-channel colonoscope for carcinoid tumors of the rectum. Dis Colon Rectum. 1996; 39:1438–1439. PMID: 8969673.

32. Higaki S, Nishiaki M, Mitani N, Yanai H, Tada M, Okita K. Effectiveness of local endoscopic resection of rectal carcinoid tumors. Endoscopy. 1997; 29:171–175. PMID: 9201465.

33. Koyama N, Yoshida H, Nihei M, Sakonji M, Wachi E. Endoscopic resection of rectal carcinoids using double snare polypectomy technique. Dig Endosc. 1998; 10:42–45.

34. Nagai T, Torishima R, Nakashima H, et al. Saline-assisted endoscopic resection of rectal carcinoids: cap aspiration method versus simple snare resection. Endoscopy. 2004; 36:202–205. PMID: 14986216.

35. Kim YJ, Lee SK, Cheon JH, et al. Efficacy of endoscopic resection for small rectal carcinoid: a retrospective study. Korean J Gastroenterol. 2008; 51:174–180. PMID: 18451691.
36. Zhou PH, Yao LQ, Qin XY, et al. Advantages of endoscopic submucosal dissection with needle-knife over endoscopic mucosal resection for small rectal carcinoid tumors: a retrospective study. Surg Endosc. 2010; 24:2607–2612. PMID: 20361212.

37. Park HW, Byeon JS, Park YS, et al. Endoscopic submucosal dissection for treatment of rectal carcinoid tumors. Gastrointest Endosc. 2010; 72:143–149. PMID: 20381798.

38. Berkelhammer C, Jasper I, Kirvaitis E, Schreiber S, Hamilton J, Walloch J. "Band-snare" resection of small rectal carcinoid tumors. Gastrointest Endosc. 1999; 50:582–585. PMID: 10502190.

39. Moon JH, Kim JH, Park CH, et al. Endoscopic submucosal resection with double ligation technique for treatment of small rectal carcinoid tumors. Endoscopy. 2006; 38:511–514. PMID: 16767589.

40. Ishikawa H, Imanishi K, Otani T, Okuda S, Tatsuta M, Ishiguro S. Effectiveness of endoscopic treatment of carcinoid tumors of the rectum. Endoscopy. 1989; 21:133–135. PMID: 2743944.

41. Koura AN, Giacco GG, Curley SA, Skibber JM, Feig BW, Ellis LM. Carcinoid tumors of the rectum: effect of size, histopathology, and surgical treatment on metastasis free survival. Cancer. 1997; 79:1294–1298. PMID: 9083149.
Fig. 1
Conventional polypectomy. (A) There is a 6 mm-sized yellow colored submucosal tumor at rectum. (B) Submucosal injection is done. (C) Snaring of the elevated submucosal lesion is done. (D) It shows a post-polypectomy ulcer.
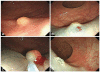
Fig. 2
Endoscopic submucosal resection with band ligation. (A) There is a 6 mm-sized yellow colored submucosal mass at rectum. (B) Tumor is aspirated by band ligation cap. (C) Tumor is ligated by band. (D) Snaring of the ligated tumor below band is done.
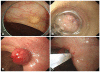
Table 1
Demographic Characteristics and Endoscopic Findings in Patients with Rectal Carcinoid Resected by Polypectomy and ESMR-L




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download