Abstract
A submucosal gastric adenocarcinoma, especially the signet ring cell type, is rare. The histologic evaluation techniques for this lesion has not been established; however, histologic confirmation is very important for decision of treatment method. Here, we report a 57-year-old man with a 12-cm gastric submucosal signet ring cell type adenocarcinoma, diagnosed by an endoscopic ultrasound-guided Trucut biopsy and immunochemical studies. This case suggests that the endoscopic ultrasound-guided Trucut biopsy might be a useful diagnostic method in cases of gastric adenocarcinoma with features of gastrointestinal stromal tumor.
Gastric cancer with the appearance of a submucosal tumor is rare. Histologic confirmation of this lesion is difficult, although it is very important for decision of treatment method.1 Various methods, including endoscopic ultrasound-guided fine needle aspiration (EUS-FNA),2,3 EUS-guided trucut biopsy (EUS-TCB), endoscopic submucosal-mucosal resection (ESMR),4 and open surgery have been reported to be used for confirmation of the diagnosis.5 However, the best method for tissue acquisition has not been established yet. Here, we report a case with a 12 cm submucosal signet ring cell type adenocarcinoma, diagnosed by EUS-TCB and immunochemical studies.
A 57-year-old man presented with epigastric pain and a 6 kg weight loss over 3 months. The physical examination revealed no significant abnormalities. Laboratory tests at admission showed white blood cell count of 13,050/mm3 (normal range, 4,800 to 10,800), hemoglobin 14.2 g/dL (13 to 18), carcinoebryonic antigen 1.5 ng/mL (0 to 5), and CA19-9 107.3 U/mL (0 to 36). The endoscopy showed an intraluminal protruding lesion covered with normal mucosa along the anterior wall of the lower body, antrum, and duodenal bulb (Fig. 1). A computed tomography of the abdomen showed about a 10×12 cm exophytic mass at the antrum and lower body of the stomach, with invasion to the liver, pancreas, and transverse colon (Fig. 2).
EUS with a radial endoscope (UE 260; Olympus, Tokyo, Japan) was performed subsequently to assess the gastric wall and evaluate the characteristics of the mass. The EUS showed a heterogeneous echogenic texture with multiple hyperechoic deposits and anechoic necrotic zones inside the large tumor mass that was thought to have developed in the fourth hypoechoic layer (muscularis propria). However, the mucosal and submucosal layer were intact, and the extraluminal border could not be assessed due to the very large size of the mass (Fig. 3).
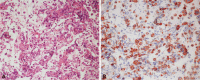
A non-operable malignant gastrointestinal stromal tumor (GIST) was first suspected. Therefore, EUS-TCB with a linear endoscope (UCT240; Olympus) was performed for rapid pathology to confirm the clinical suspicion with immunohistochemical staining and to save costs. The histopathology revealed numerous infiltrating signet ring cells (H&E stain, ×200). Since surgical treatment was not possible, the patient was treated with chemotherapy. The signet ring tumor cells were immunoreactive for cytokeratin (CK, ×200) (Fig. 4). Chemotherapy with TS-1 (TS-ONE; Jeil Pharmaceutical Co., Seoul, Korea) plus IV cisplatin (CISPLATIN; Ildong Pharmaceutical Co., Seoul, Korea) was started. TS-1 was given orally at a dose of 40 mm/m2 twice daily for 2 weeks followed by a 1-week rest, and cisplatin was given intravenously on day 1 at a dose of 60 mg/m2. During the admission, a percutaneous drainage (PCD) tube was inserted into the abdomen due to increased ascites. The ascites cytology revealed metastatic adenocarcinoma. Bleeding around the PCD site occurred. The general condition of the patient deteriorated rapidly after about 2 weeks from admission and the patient died 2 months after the diagnosis.
This case report of a patient with gastric primary signet-ring cell carcinoma with features of GIST was confirmed by EUS-TCB. Gastric cancers have various endoscopic findings, and histology is used to confirm the diagnosis.6 However, it is often difficult to confirm the histologic diagnosis despite taking a biopsy specimen, because the tumor surface of gastric cancers mimicking a submucosal tumor (GCSMT) is covered by normal mucosa.1 GCSMTs are very rare; they account for 0.1% to 0.63% of all resected gastric cancers.7 Moreover, the histological confirmation of a signet ring cell type is uncommon. EUS alone can provide useful information on GCSMTs;8 however, it is difficult to determine the histological nature of the lesions from the EUS image alone.9 The methods used to overcome this problem include EUS-FNA,2,3 EUS-TCB, ESMR,4 laparoscopic excision biopsy,10 and open surgery.5 However, the best method for confirmation of the diagnosis has not been established yet. For a definite diagnosis of a submucosal tumor, tissue acquisition, and pathology confirmation are usually required. Recently, Mekky et al.11 reported that the results of EUS-FNA in 141 patients with gastric SMTs were diagnostic, suspicious, and nondiagnostic in 43.3%, 39%, and 17.7% of cases, respectively, with an overall accuracy of 95.6% and the accuracy of differentiating potentially malignant lesions of 94.2%. Săftoiu et al.12 reported that the yield of adequate tissue sampling was similar for EUS-FNA and EUS-TCB (96.4% vs. 89.3%, p=not significant). However, the accuracy for obtaining a specific diagnosis was significangly lower for the EUS-FNA compared to the EUS-TCB (5.3% and 68.4%, p<0.005). Cantor et al.13 suggested that the ESMR has a significantly higher diagnostic yield than jumbo forceps biopsy with the use of the bite-on-bite technique for the evaluation of subepithelial lesions limited to the submucosa. The diasgnositc yield of the jumbo forceps biopsy was four out of 23 cases (17%) compared to 20 out of 23 cases (87%) of the ESMR (p<0.001). The ESMR, however, accompanies major complications such as bleeding (0% to 24%) and perforation (0% to 5%). Therfore, the EUS-TCB was performed in this case for rapid pathology in order to confirm the clinical suspicion with immunohistochemical staining, with a relatively lower complication rate.
In conclusion, this is the first report of the EUS-TCB used as a diagnostic tool in a case of gastric primary signet-ring cell carcinoma with features of GIST. EUS-TCB has low complication rate and enables immunohistochemical staining unlike other methods such as EUS-FNA, ESMR, or open surgery, which is why it should be considered when results of a mucosal biopsy are not diagnostic. The possibility of signet ring cell carcinoma, which has a poor prognosis, should always be considered even in cases with GCSMT developing in the fourth gastric layer.
References
1. Wiersema MJ, Wiersema LM, Khusro Q, Cramer HM, Tao LC. Combined endosonography and fine-needle aspiration cytology in the evaluation of gastrointestinal lesions. Gastrointest Endosc. 1994; 40(2 Pt 1):199–206. PMID: 8013822.

2. Kume K, Yoshikawa I, Yamazaki M, Abe S, Murata I, Otsuki M. A case of gastric cancer with features of submucosal tumor. Gastrointest Endosc. 2001; 53:247–249. PMID: 11174309.

3. Gu M, Ghafari S, Nguyen PT, Lin F. Cytologic diagnosis of gastrointestinal stromal tumors of the stomach by endoscopic ultrasound-guided fine-needle aspiration biopsy: cytomorphologic and immunohistochemical study of 12 cases. Diagn Cytopathol. 2001; 25:343–350. PMID: 11747229.

4. Kim SY, Park JJ, Cho Y, et al. A case of submucosal tumor-like early gastric adenocarcinoma diagnosed by endoscopic mucosal resection. Korean J Gastrointest Endosc. 2005; 31:404–408.
5. Takahashi T, Otani Y, Yoshida M, et al. Gastric cancer mimicking a submucosal tumor diagnosed by laparoscopic excision biopsy. J Laparoendosc Adv Surg Tech A. 2005; 15:51–56. PMID: 15772477.

6. Matsushita M, Shiroeda O, Inokuchi H, et al. A case of early gastric cancer manifesting as a submucosal tumor. Dig Endosc. 1994; 6:269–274.

7. Takemoto N, Baba Y, Kaku Y, et al. Radiologic diagnosis of gastric cancer morphologically mimicking submucosal tumor. Stomach Intest. 1995; 30:759–768.
8. Nakamura T, Suzuki T, Kobayashi S, et al. Diagnosis of gastric carcinoma with the appearance of a submucosal tumor of the stomach, using endoscopic ultrasonography. Stomach Intest. 1995; 30:787–798.
9. Lee JH, Shin JE, Kim BH, et al. A case of early gastric adenocarcinoma mimiking a submucosal tumor. Korean J Gastrointest Endosc. 2007; 35:250–253.
10. Yasuda K, Nakajima M, Yoshida S, Kiyota K, Kawai K. The diagnosis of submucosal tumors of the stomach by endoscopic ultrasonography. Gastrointest Endosc. 1989; 35:10–15. PMID: 2646166.

11. Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010; 71:913–919. PMID: 20226456.
12. Săftoiu A, Vilmann P, Guldhammer Skov B, Georgescu CV. Endoscopic ultrasound (EUS)-guided Trucut biopsy adds significant information to EUS-guided fine-needle aspiration in selected patients: a prospective study. Scand J Gastroenterol. 2007; 42:117–125. PMID: 17190771.

13. Cantor MJ, Davila RE, Faigel DO. Yield of tissue sampling for subepithelial lesions evaluated by EUS: a comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest Endosc. 2006; 64:29–34. PMID: 16813799.

Fig. 1
Initial endoscopic view showing the intraluminal protruding lesion covered by benign appearing mucosa along the anterior wall of the lower body, antrum, and the duodenal bulb.
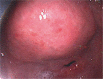
Fig. 2
A computed tomography of the abdomen, showing about a 10×12 cm exophytic mass (arrow) at the antrum and lower body of stomach with invasion of the liver, pancreas, and transverse colon.
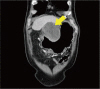
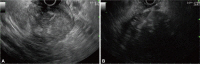
Fig. 3
(A) Endoscopic ultrasound (EUS) imaging showing heterogeneous echo texture with hyperechoic deposits and anechoic necrotic zones inside a large tumor mass suspected to have developed in the fourth hypoechoic layer (muscularis propria). The extraluminal border could not be assessed due to the very large size of the mass. (B) The QuickCore needle (19 gauge) is advanced under EUS guidance to mass.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download