Abstract
Background/Aims
The rapid urease test (RUT) is an invasive method to diagnose Helicobacter pylori infection, which relies on the acquisition and examination of gastric antrum and body tissues. We determined and compared the efficacy of RUT when the tissues were examined separately or after being combined.
Methods
Two hundred and fourteen patients were included and underwent esophagogastroduodenoscopy from July 2008 to June 2010. The separate test was defined as evaluating the status of infectivity of H. pylori from the antrum and body separately; whereas the united test was carried out putting both tissues from the antrum and body in the same RUT kit. All RUTs were read by a single observer 1, 3, 6, 12, and up to 24 hours later. We also got two biopsy specimens stained with hematoxylin and eosin and quantified H. pylori density was calculated on a scale of 0 to 3.
Results
Overall positivity for H. pylori was 137 (64%) for the separate test and 148 (69.2%) for the united test (p<0.01). The mean time to a positive test was 3.58 hours for the separate test and 1.69 hours for the united test (p<0.01). The correlation between the time to positive RUT and the severity of histology showed r=+0.556 for the antrum (p<0.01) and r=+0.622 for the body (p<0.01).
Helicobacter pylori is a definitive cause of atrophic gastritis, gastric and duodenal ulcers, and duodenitis. More ominously, the presence of the bacterium is linked with pathogenesis of gastric cancer and mucosal lymphoid tissue lymphoma.1
Confirmation of H. pylori is an important step to the treatment and prevention of these diseases. The rapid urease test (RUT), which was developed by Barry Marshall and was made commercially available by Kimberly-Clark, is based on the detection of urease.2 The test is a fast, accurate, and inexpensive means of diagnosing H. pylori infection. Typically during routine endoscopy, the test is performed on the antrum and body tissues individually, rather than on combined tissues. The sensitivity of the RUT can be increased and the time to achieve a positive results decreased by increasing the amount of biopsied specimens.2,3 However, the influence of examining combined tissue samples rather than individual testing has not been explored. We evaluated the usefulness of the united RUT compare to traditional, separate test for the detection of H. pylori. The present study used RUT to examine individual antrum and body tissue samples as well as combined samples. In addition, histologic staining of the tissues was performed to explore whether the time to attain a positive result correlated with H. pylori population density.
Between July 2008 and June 2010, patients who underwent an esophagogastroduodenoscopy at Gyeongsang National University Hospital as part of a general check-up or in response to gastrointestinal symptoms including dyspepsia, abdominal pain, and heartburn were enrolled in this prospective study. Exclusion criteria were previous upper gastrointestinal surgery; suspected pernicious anemia; previous H. pylori eradication treatment; use of proton pump inhibitors, H2-receptor antagonists, bismuth salts, or antibiotics in the preceding 4 weeks; and use of a concomitant anticoagulant. This study was approved by the Clinical Research Ethics Committee of Gyeongsang National University Hospital and all patients provided a written informed consent before the procedure.
During each endoscopy, four antrum and body biopsy specimens were obtained. Gastric antral biopsies were taken from the prepyloric region, within 1 to 3 cm of the pylorus, while the body biopsies were taken from the middle of the greater curvature. Each sample was acquired using sterilized standard-sized biopsy forceps (Biopsy forcep 061512101; MTW, Wesel, Germany). Of the four samples acquired from each site, two were used for the separate and united RUT (ASAN Easy Test H. pylori; Asan Pharmaceutical, Seoul, Korea), and the remaining two were used for histological staining. Different biopsy forceps were used to minimize the possibility of influence from one site to the other. Separate testing was routinely performed to evaluate the results from each different site; a result was considered positive (gel pellet turned dark pink) if at least one RUT procedure was positive. United testing involved examination of combined tissue samples in the same test. All RUT chambers were incubated at room temperature, and an investigator blinded to the sample sites and number of biopsies recorded results at 1, 3, 6, and 12 hours, and at intervals thereafter up to 24 hours (Fig. 1). For histological examinations, two biopsy specimens were placed each in 10% buffered formalin saline fixative and stained with hematoxylin and eosin. Stained specimens were evaluated by qualified general histopathologists who did not have access to accompanying routine clinical information. H. pylori histological density quantification was performed on a 0 to 3 scale (0 being none and 3 being severe) using the Updated Sydney System4 without knowledge of the RUT result.
Data were analyzed using SPSS software 12.0 (SPSS Inc., Chicago, IL, USA). McNemar's test was used to assess the difference between the two sample routines and the Pearson chisquare test was used to evaluate the correlation between the time to positivity and histologic H. pylori density grade. A p-value of <0.05 was considered statistically significant.
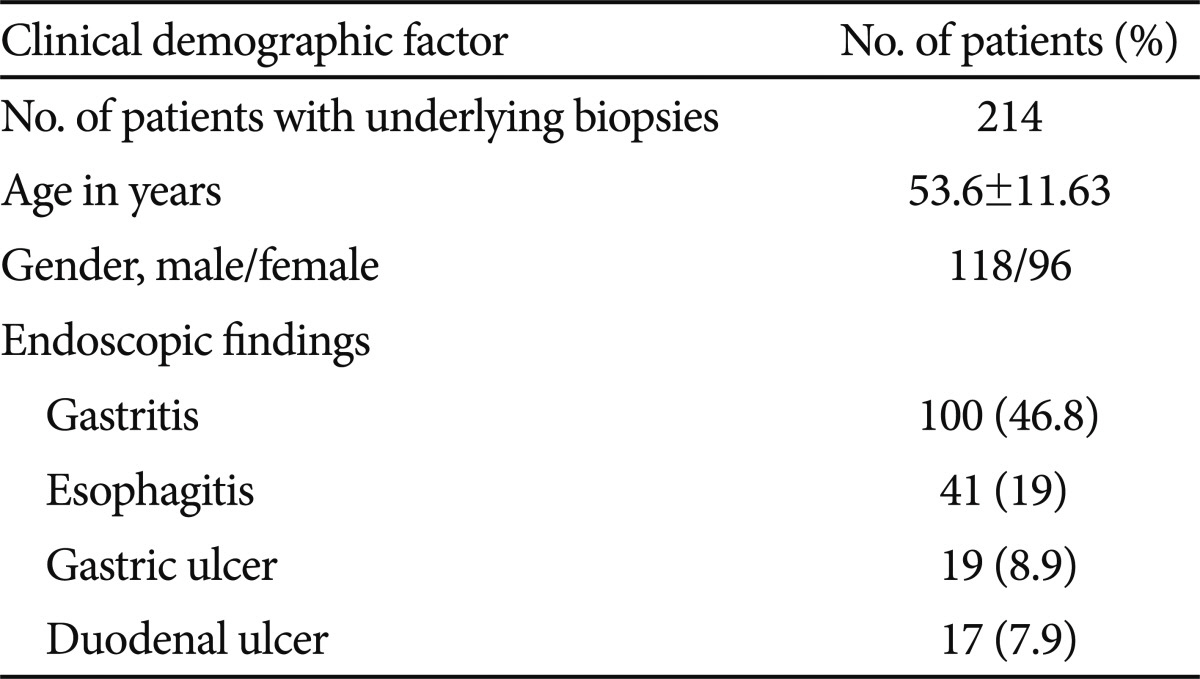
A total of 214 patients (118 males, 96 females; mean age, 53.6 years; range, 21 to 78 years) were included in this prospective study. There were no complications during the endoscopic procedure. Gastritis was evident in 46.8% of the subjects, followed by esophagitis (19%), gastric ulcer (8.9%), and duodenal ulcer (7.9%) (Table 1).
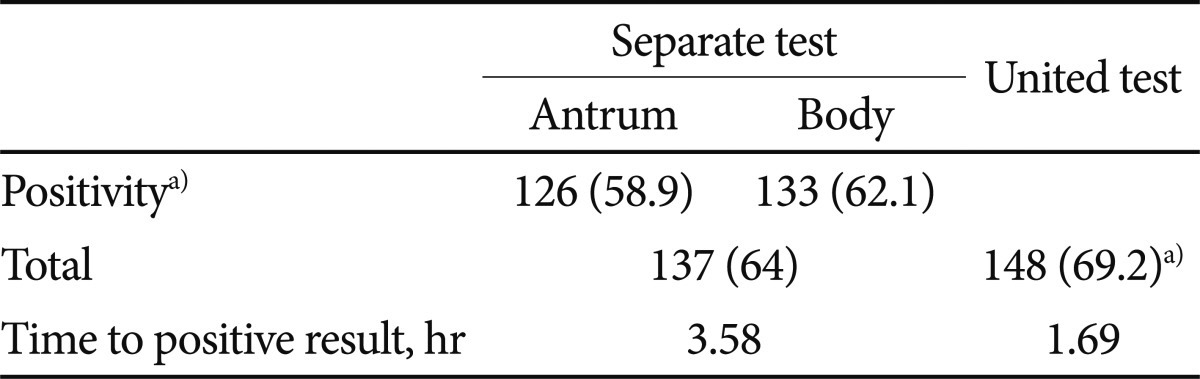
Of the 214 subjects, 137 (64%) were positive on the separate RUT (58.9% in the antrum, 62.1% in the body), while 148 (69.2%) were positive on the united RUT (p<0.01) (Table 2). All negative united RUT results were also negative on the separate RUT, and 11 cases with negative separate RUT results were positive on the united RUT (Table 3). In 8 of these 11 cases, it took 24 hours to get positive results.
Fig. 2 shows the cumulative percentage of positive urease test between the separate and united tests 1, 3, 6, 12, and 24 hours later. The overall proportion of positive RUT at each time point was consistently higher in the united RUT group (p<0.01).
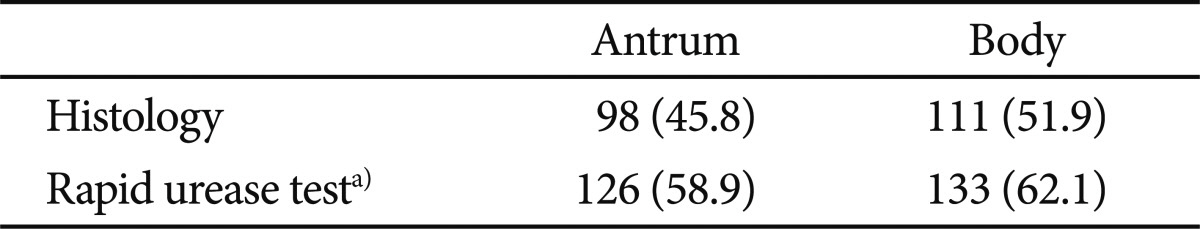
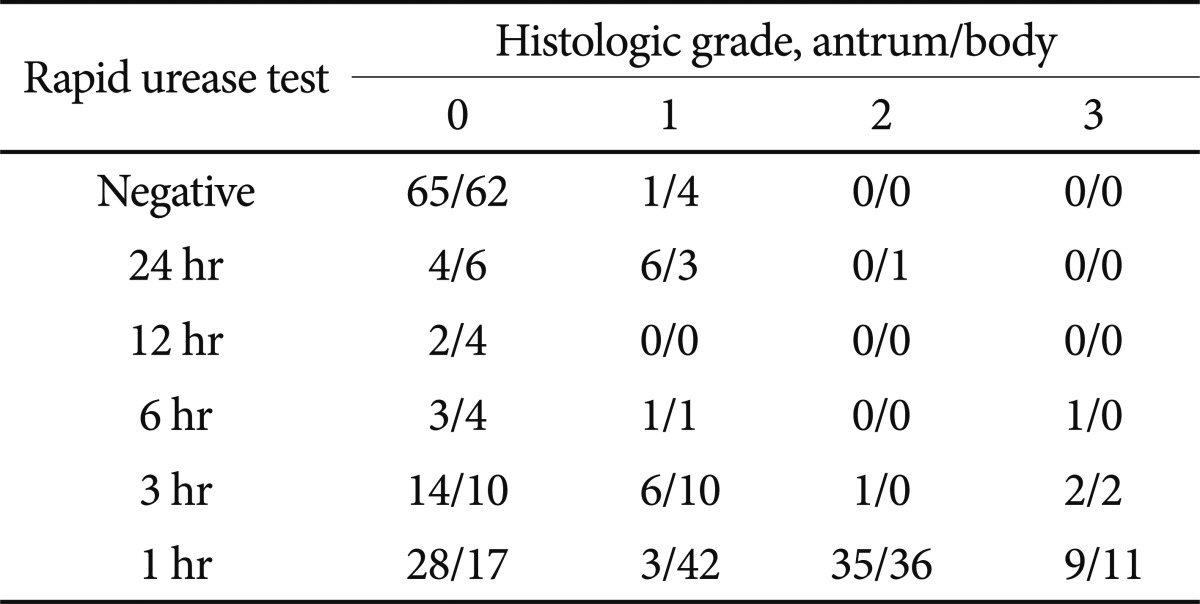
Positivity in the united RUT procedure was significantly higher than histology (69% vs. 60.2%, respectively; p<0.01) (Table 4). No significant difference was discernible between the separate RUT and histology. The correlation between the time to positive RUT and the severity of histology showed r=+0.556 for the antrum (p<0.01) and r=+0.622 for the body (p<0.01) (Table 5).
Accurate initial diagnosis of H. pylori infection and confirmation of eradication as a result of therapy are crucial, in light of the severe and even life-threatening consequences of infection. In patients with peptic ulcer disease, eradication can prevent recurrence and can change natural history of ulcer.5 Methods to detect H. pylori include non-invasive serologic examination using stool, saliva, and urine; invasive biopsy based urease test; cultural detection; and histology. Nowadays, RUT, which can be conducted as a biopsy-based invasive method, is feasible for rapid, accurate, and convenient detection of microorganism in the stomach.6 It is the gel-based test utilizing the pH indicator phenol red. If H. pylori urease is present in the biopsy specimen, the phenol red-containing gel changes from yellow to dark pink in response to the urease-driven pH change.
An ongoing debate has centered on the proper sites and numbers of gastric biopsies for the diagnosis of H. pylori. In one study, examination of 12 different sites in the stomach reported the highest yields of H. pylori from antral sites of the greater and lesser curvature.7 Another study reported an increased RUT sensitivity when two biopsies were taken, one from the antrum and one from the body.8 The mid-body may be the most reliable site for diagnosis.9
To this point, the assumption has been that separate biopsies from the antrum and body represent the prudent course in diagnosis of H. pylori infections. On the other hand, the influence of increasing numbers of biopsy samples in the same RUT chamber has been investigated. Results obtained with the testing of doubled tissue samples have been increased sensitivity and hastened development of the positive test.10,11
In our study, the positivity of H. pylori in the body tissue samples was higher than that of the antrum samples both histologically and by RUT. Our observations echo those of a previous study,12 in which increasing atrophy of the antrum was associated with decreasing H. pylori density of the antrum itself. Additional support comes from the present finding that the majority of the enrolled subjects (79.9%) exhibited an atrophic change in the antrum tissues, where colonization of H. pylori does not occur. Combining different stomach sites yields better RUT results, likely because this strategy better accounts for the patchy distribution of H. pylori, especially in patients with severe atrophic or metaplastic change.
Our study, for the first time to our knowledge, correlated the rapidity of the RUT with increasing histological detection of H. pylori. It seems that the increased amount of urease in the medium augmented by the united test hastens the development of a positive reaction. It is conceivable, therefore, that the test time could be exploited as an indicator of the severity of H. pylori infection and a gauge to the success of eradication regimens. If so, this may address the high false negativity noted after eradication of H. pylori when the titer number of the bacterium declines.13 Combined testing would carry additional advantages of time-saving and cost-saving by reducing the use of RUT kit.
This study had several limitations. First, the results of this study could include some false positivity and there were no sensitivity and specificity compared to golden standard test. Histologic examination via modified Giemsa stain was not adopted, which is the method of choice in diagnosing H. pylori because of its high sensitivity, easy performance, and reproducibility.14 Furthermore, we could not perform the urea breath test, which is an indicator of the presence of bacteria irrespective of different disposition of H. pylori with higher sensitivity than histology. Also, only two specimens could be simultaneously tested in the RUT kit because the dimensions of the test chamber prevented addition of any more material. Development of RUT kits with larger sample capacity will be welcomed.
While the united test group was not compared with multi-biopsy specimens from the antrum and body, we believe that further study is warranted to seek best combination of multiple tissues and various sites of the stomach including angle, high body, and fundus in confirming H. pylori. During endoscopy conducted to obtain tissues from normal-looking mucosa, intentionally avoiding atrophic or metaplastic lesions may result in an improved diagnostic yield of H. pylori.
In conclusion, our study shows that combining tissues from different sites of the stomach is superior to separate testing in terms of sensitivity and the time to achieve positive results. These diagnostic advantages are complimented by the cost-savings with reduced use of the test kits.
References
1. Marshall BJ. Helicobacter pylori. Am J Gastroenterol. 1994; 89(8 Suppl):S116–S128. PMID: 8048402.
2. Siddique I, Al-Mekhaizeem K, Alateeqi N, Memon A, Hasan F. Diagnosis of Helicobacter pylori: improving the sensitivity of CLOtest by increasing the number of gastric antral biopsies. J Clin Gastroenterol. 2008; 42:356–360. PMID: 18277905.
3. Vassallo J, Hale R, Ahluwalia NK. CLO vs histology: optimal numbers and site of gastric biopsies to diagnose Helicobacter pylori. Eur J Gastroenterol Hepatol. 2001; 13:387–390. PMID: 11338067.
4. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996; 20:1161–1181. PMID: 8827022.
5. Graham DY, Lew GM, Klein PD, et al. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer: a randomized, controlled study. Ann Intern Med. 1992; 116:705–708. PMID: 1558340.
6. Marshall BJ, Warren JR, Francis GJ, Langton SR, Goodwin CS, Blincow ED. Rapid urease test in the management of Campylobacter pyloridisassociated gastritis. Am J Gastroenterol. 1987; 82:200–210. PMID: 3548326.
7. Genta RM, Graham DY. Comparison of biopsy sites for the histopathologic diagnosis of Helicobacter pylori: a topographic study of H. pylori density and distribution. Gastrointest Endosc. 1994; 40:342–345. PMID: 7794303.

8. Weston AP, Campbell DR, Hassanein RS, Cherian R, Dixon A, McGregor DH. Prospective, multivariate evaluation of CLOtest performance. Am J Gastroenterol. 1997; 92:1310–1315. PMID: 9260796.
9. Satoh K, Kimura K, Taniguchi Y, et al. Distribution of inflammation and atrophy in the stomach of Helicobacter pylori-positive and -negative patients with chronic gastritis. Am J Gastroenterol. 1996; 91:963–969. PMID: 8633589.
10. Laine L, Chun D, Stein C, El-Beblawi I, Sharma V, Chandrasoma P. The influence of size or number of biopsies on rapid urease test results: a prospective evaluation. Gastrointest Endosc. 1996; 43:49–53. PMID: 8903818.

11. Lim LL, Ho KY, Ho B, Salto-Tellez M. Effect of biopsies on sensitivity and specificity of ultra-rapid urease test for detection of Helicobacter pylori infection: a prospective evaluation. World J Gastroenterol. 2004; 10:1907–1910. PMID: 15222034.
12. Enomoto H, Watanabe H, Nishikura K, Umezawa H, Asakura H. Topographic distribution of Helicobacter pylori in the resected stomach. Eur J Gastroenterol Hepatol. 1998; 10:473–478. PMID: 9855062.

13. Shimoyama T, Fukuda Y, Fukuda S, Munakata A, Yoshida Y. Validity of various diagnostic tests to evaluate cure of Helicobacter pylori infection. J Gastroenterol. 1996; 31:171–174. PMID: 8680535.
14. Rotimi O, Cairns A, Gray S, Moayyedi P, Dixon MF. Histological identification of Helicobacter pylori: comparison of staining methods. J Clin Pathol. 2000; 53:756–759. PMID: 11064668.

Fig. 2
Cumulative percentage of positive rapid urease test (RUT) test (CLOtest) between united and separate test according to time to results (p<0.01 for comparison between united and separate test in all time measured).





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download