Abstract
Endoscopic resection has been accepted as both minimally invasive and curative treatment modality for early gastric cancer (EGC). The widely accepted indication of endoscopic resection for EGC is small sized, differentiated mucosal cancer in which the risk of lymph node metastasis is negligible. Tumor size can be measured by conventional endoscopy, and chromoendoscopy, magnifying endoscopy, narrow band imaging, autofluorescence imaging can also be helpful for accurate estimation of tumor size. Pretreatment tumor histology can be assessed with endoscopic biopsy, and also be measured by confocal endomicroscopy (so called "virtual biopsy"). Although endoscopic ultrasonography may be helpful for the assessment of tumor depth in EGC, the accurate assessment of tumor depth can be performed by the typical findings in the conventional endoscopy, by which treatment modality can be decided according to the depth of tumor invasion.
Currently endoscopic resection for early gastric cancer (EGC) has become the established treatment of choice in Eastern countries including Korea and Japan because it is not only minimally invasive but also an effective therapy in the management of EGC without risk of lymph node metastasis.1,2 Endoscopic treatment for curative intent is only indicated for either mucosal or minute submucosal invasive, small sized and differentiated tumor as lymph node metastases are rare.3 Therefore, successful endoscopic resection requires accurate tumor size estimation, mandatory prediction of the depth of tumor invasion, and identification of lymph nodal metastasis.
The established indications for endoscopic resection of EGC are differentiated tumors of less than 3 cm in the absence of ulceration and lymphovascular invasion because these tumors rarely metastasize to the lymph nodes.4 One study reported that there was a significant correlation between tumor size larger than 3 cm and an increased risk of lymph node metastasis.3 Thus, gastric tumor size is fundamental in the selection of patients suitable for endoscopic resection.
Conversely, the size criteria for endoscopic resection were based heavily on pathologic evaluation after surgery;3,4 tumor size was measured in the post formalin fixation specimen. In contrast, at the time of endoscopy, endoscopists estimate only approximate tumor size on the basis of endoscopic imaging, which may cause measurement discrepancy between endoscopic estimation and pathologic measurements.
Endoscopic measurement of tumor size can be enhanced by chromoendoscopy, magnifying endoscopy with/without narrow band imaging or autofluorescence imaging. In cases of large tumor size or undifferentiated histology, there is a possibility of under-estimation of tumor size, which may be an important factor of incomplete resection of EGC.
Endoscopic ultrasound (EUS) has been widely utilized in the locoregional staging of gastroesophageal tumors and regarded as highly accurate, thereby making it the first choice imaging modality in depth of tumor (T) staging.5,6 However, enthusiasm for accurate prediction using EUS for T classification has been tempered by the demonstration that correct staging rates of EUS in differentiating between the mucosa and beyond the mucosa were tremendously heterogeneous, indicating an unacceptably low accuracy for the submucosal invasive tumor.7,8 Although previous studies have revealed similar staging accuracy between EUS and conventional endoscopy, EUS has a significantly higher overstaging rate than endoscopy.8,9 Furthermore, when EUS examination was performed in a strictly blinded manner without information of endoscopic assessment, staging results were disappointing.10
To assess the accuracy of conventional endoscopy for T classification in EGC using pathological staging as the gold standard, a total of 2,105 patients with endoscopically suspected EGC were enrolled in the study.11 Endoscopic criteria for penetration depth (mucosa or submucosa) were determined by characteristic endoscopic features based on the regularity/nodularity/granularity of surface, marginal elevation, erosion or ulcer, tumor size in protruding type, and shape of fold convergence. Staging was retrospectively conducted by consensus between two endoscopists and was compared with pathological staging as the gold standard. Clinicopathological factors affecting accuracy were also evaluated.
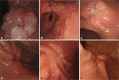
Endoscopic features of intramucosal cancer were defined as smooth surface protrusion, shallow and even erosion, smooth marginal elevation, flat or superficial spreading lesion, smooth tapering of fold convergence in depressed lesion, and tumor size less than 3 cm in protruding type (Fig. 1). On the other hand, endoscopic features of submucosal cancer were defined as irregular or nodular surface, irregular protrusion without flexibility, deep ulcer with marked marginal elevation, converging fold with clubbing, abrupt cutting or fusion, and tumor size more than 3 cm in protruding type (Fig. 2).
In clinical characteristics, depressed type was the main feature in 79.5%, and ulcer was not combined in 85.7%. The proportion of differentiated histology was 66.1%, and the tumor was confined in mucosa in 58.1%. Overall accuracy of endoscopic staging was 78.0%. Sensitivity and positive predictive value for T1m was 85.5% and 82.0%, whereas for T1sm the rates was lower at 72.6% and 71.9% (p=0.001). If T2 was included in the beyond the mucosal invasion, sensitivity and positive predictive value for T1sm, and overall accuracy increased up to 73.9%, 78.5%, and 80.6%, respectively. The rates for understaging and overstaging were 13.5% and 8.5% respectively. Accuracy was lower for flat/depressed configuration, larger tumor size, or submucosal invasion.
References
1. Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1,000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009; 69:1228–1235. PMID: 19249769.

2. Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009; 58:331–336. PMID: 19001058.

3. Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000; 3:219–225. PMID: 11984739.

4. Yamao T, Shirao K, Ono H, et al. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996; 77:602–606. PMID: 8616749.

5. Botet JF, Lightdale CJ, Zauber AG, et al. Preoperative staging of gastric cancer: comparison of endoscopic US and dynamic CT. Radiology. 1991; 181:426–432. PMID: 1924784.

6. Pfau PR, Perlman SB, Stanko P, et al. The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc. 2007; 65:377–384. PMID: 17321235.

7. Kwee RM, Kwee TC. The accuracy of endoscopic ultrasonography in differentiating mucosal from deeper gastric cancer. Am J Gastroenterol. 2008; 103:1801–1809. PMID: 18564110.

8. Yanai H, Noguchi T, Mizumachi S, et al. A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut. 1999; 44:361–365. PMID: 10026321.

9. Yanai H, Matsumoto Y, Harada T, et al. Endoscopic ultrasonography and endoscopy for staging depth of invasion in early gastric cancer: a pilot study. Gastrointest Endosc. 1997; 46:212–216. PMID: 9378206.

10. Meining A, Dittler HJ, Wolf A, et al. You get what you expect? A critical appraisal of imaging methodology in endosonographic cancer staging. Gut. 2002; 50:599–603. PMID: 11950802.

11. Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc. 2011; 73:917–927. PMID: 21316050.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download