Abstract
A submucosal lesion, more appropriately a subepithelial lesion, is hard to diagnose. Endoscopic ultrasonography is good to differentiate the nature of submucosal lesion. For definite diagnosis, tissue acquisition from submucosal lesion is necessary, and many methods have been introduced for this purpose mainly by endoscopic ultrasonography, such as endoscopic ultrasound-guided fine needle aspiration (EUS-FNA), EUS-guided Trucut Biopsy (TCB), and EUS-guided fine needle biopsy (FNB). For EUS-FNA, adequate processing of specimen is important, and for proper diagnosis of EUS-FNA specimen, both cytologic and histologic examinations, including immunohistochemical stains, are important. All gastrointestinal stromal tumors have some degree of malignant potential, so there have been a lot of efforts and methods to increase diagnostic yields of submucosal lesion. We herein review the current hot topics on EUS-FNA for submucosal tumor, such as needles, on-site cytopathologists, immunohistochemical stains, EUS-TCB, EUS-FNB, Ki-67 labelling index, DOG1, and combining EUS-FNA and EUS-TCB.
A submucosal lesion is located from deep mucosa to deeper serosa, so more accurate term is a subepithelial lesion.1 Gastric submucosal lesion is found in about 0.36% of cases by upper gastrointestinal endoscopies.2
When a submucosal lesion is suspected at endoscopy, the first step is to differentiate it from extramural compression, and endoscopic ultrasonography (EUS) is the most accurate tool for this job. The second step is to determine the nature of the submucosal tumor (SMT), for which also EUS is the most accurate tool. EUS shows typical findings for lipoma, duplication cyst and pancreatic nest,1 but in hypoechoic lesions, such as leiomyomas, gastrointestinal stromal tumors (GISTs) and schwannomas, EUS findings are not enough for definite diagnosis, which is why we need tissue acquisition from the submucosal lesion. Because it is located deeper than epithelium, we cannot take specimens from the subepithelial lesion using conventional endoscopic biopsy method. Various methods, such as bite-on-bite technique, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA), EUS-guided Trucut biopsy (TCB), and EUS-guided fine needle biopsy (FNB), have been introduced to obtain samples of sub-epithelial lesion.
For gastrointestinal subepithelial lesions, bite-on-bite technique of two to eight bites using conventional-sized forceps yielded 38% of diagnostic rate (54% in the esophagus, 28% in the stomach and duodenum; p<0.019).3
For SMTs, cytology, cell block processing, and immunohistochemical stains are available using 22-gauge EUS-FNA needle.4 Immunohistochemical stains are helpful for cytopathologic differentiations. Mitotic counts and immunohistochemical stains usually cannot be performed on smears, and so it requires cell blocks.2 Cytological subclassification of submucosal spindle cell lesions by immunohistochemical staining on cell blocks correlate very well with the final diagnosis.5
All GISTs have some degree of malignant potential.6 Even small GISTs may present malignant features on histologic examination or biologic behavior. A lot of efforts have been made to increase the diagnostic yields of submucosal lesions, but each method has their own limitations.
EUS-FNA, first reported by Vilmann et al.7 in 1992, can obtain cell block specimen for histologic examinations and immunohistochemical staining. When processing EUS-FNA specimen, one slide is fixed by air drying, stained with modified Giemsa (Diff-Quick; Baxter Diagnostics, Inc. McGaw Park, IL, USA) and examined on-site for specimen adequacy. The other slides are fixed in the alcohol and stained using the Papanicolaou technique. Cell block material is fixed in 10% formalin, embedded in paraffin, cut thinly and then stained with hematoxylin and eosin. Diagnosis is done with smear cytology, histology, and immunohistochemical staining of the cell blocks.4 Yoshida et al.8 proposed several preparation methods of EUS-FNA specimen for good results. First, maintaining negative pressure of needle many times is necessary to get sufficient tissue because GIST cells have tight cell to cell adhesion. Second, aspirated materials should be pushed out from the needle under the saline solution so that they are not dried. Third, one drop of 1% albumin is helpful for preserving the morphology when cells are collected with cytospin. Fourth, cell degradation materials are also aspirated, especially in the presence of necrosis.
EUS findings are not sufficient to distinguish malignant and benign stromal cell tumors. EUS findings can diagnose malignant subepithelial lesions with a sensitivity of only 64% and a specificity of 80%,3 which is why a further pathologic diagnosis is needed. Echogenicity enables some differentiations between GISTs, leiomyomas, and schwannomas. It was found that GISTs have generally low but slightly higher echogenicity than the proper muscle layer. That of leiomyomas was equal to that of proper muscle layer and schwannoma showed extremely low echogenicity.9 Some EUS findings indicate increased risk of malignancy; tumors with more than 3 or 4 cm of size and irregular borders are the most important features.1 Other findings such as echogenic foci, cystic spaces, ulcerated mucosa, and lymph nodes with malignant pattern are not completely decided yet. EUS findings of high grade malignancy have 1) greater size, 2) lobulated forms, 3) irregular borders, and 4) echogenic foci more often than the low grade malignancy, but EUS is not sufficient to predict malignant pathological findings accurately.10
Inter-observer variation in EUS interpretation can be a factor contributing to the discrepancy of results. Inter-observer agreement was poor in terms of echogenicity, irregular border, presence of cystic spaces, and echogenic foci.1 There are criteria developed by combining EUS features; the criteria for predicting malignancy are tumor size >3 cm in homogeneous pattern, irregular outer margins and lymph node size >10 mm. The reported sensitivity and specificity of predicting malignancy were 64% and 80%, respectively, when meeting at least 2 of these criteria.11
Gastric tumors larger than 30 mm, with irregular borders, mucosal ulceration, and non-oval shape on EUS suggest high risk GISTs. The malignant potential of gastric SMT increases when tumor size is >30 mm; approximately 50% of cases with SMTs larger than 3 cm are malignant.12 Gastric ulcers were concomitant with SMT larger than 5 cm in 39% of cases; 37% of these patients presented with malignant tumor. Therefore, patients having both SMTs and ulcers should be closely monitored if they are not planned for surgery. Tumor size correlates with malignant potential. Tumors <2 cm in diameter without clinical signs of malignancy or complications are to be followed up at 12 month intervals conservatively.12 The current role of imaging studies for predicting malignant potential is of less importance, so we should consider that all GISTs have certain malignant potential.
Mazur and Clark13 first described GISTs in 1983 as tumors negatively stained with muscle and neuron markers. All stromal tumors of the gastrointestinal tract were classified as 1) smooth muscle type, 2) neural type, 3) combined type, and 4) uncommitted type. This definition was widely accepted and the uncommitted type has been used to describe GIST in a narrow sense.10 Because most GISTs were positive for C-KIT and CD34 staining, they were assumed to have originated from interstitial cell of Cajal. In the pathogenesis of GISTs, activation of kit signaling occurred often by mutations in the c-kit gene is the most important factor. Activation of KIT, which is the product of the c-kit gene, seems important for the growth of GISTs.10
The cytologic features of GISTs are well-known. Cytologic smear shows highly cellular spindle cells, loosely cohesive cells oriented in one direction. Tumor cells have ill-defined cytoplasmic border with high magnification. The spindle shaped nuclei are usually embedded in a cyanophilic, delicate and fibrilliary background.14
Although GIST diagnosis can be made by cytomorphologic findings, those characterizations of malignant GISTs are limited. Though mitoses can be counted on the core tissue specimens, the mitotic index determined in this way is not always representative of the whole tumor.1
Histologic criteria for malignant GISTs in hematoxylin and eosin stained EUS-FNA specimen are 1) presence of mitotic figures, 2) high cellularity, and 3) severe nuclear atypia. Because counting mitosis of EUS-FNA specimens per 50 high power fields (HPFs) is difficult, the differentiation between low grade malignancy and high grade malignancy was decided by the existence of one mitosis per 5 HPF for the EUS-FNA specimens in one study.10
Differential diagnoses of benign and malignant GISTs are difficult although diagnosis of GISTs by EUS-FNA is possible. Histologic findings such as cytologic atypia, high mitotic activity can be seen in malignant GIST. In many studies, tumor size of greater than 4 to 5 cm was considered as a predictor of the malignancy of stromal tumors.15 Prognostic factors for ma-lignancy also include mitotic index (5 mitotic figures/50 HPFs) and ulcerated, cystic, or necrotic areas within the tumor.10 Presence of mitotic cells and Ki-67 labelling index are significant predictive factors for malignant GISTs.16 The presence of c-kit mutations may suggest poor prognosis of GISTs and is strongly predictive of malignant behavior, but some malignant GISTs did not show c-kit mutations.17
Average diagnostic accuracy rate of EUS-FNA is 60% to 80% in SMTs.4 Recently Mekky et al.4 reported diagnostic utility of EUS-FNA in gastric SMTs. The sampling adequacy was 83% with average 2.5 passes. EUS-FNA results were diagnostic in 43.3%, suggestive in 39%, and nondiagnostic in 17.7%. EUS-FNA results were 95.6% accurate in differentiating potential malignant lesions. Heterogeneous echo pattern predicted sampling adequacy. Better results than previous studies were attributed to on-site cytological analysis.
Sampling adequacy increased with tumor size. There was an increase in sampling adequacy in SMT with a 95% yield for lesion size of >50 mm.4 Another study reported 100% yield of EUS-FNA with lesion size of >40 mm.18 Suzuki et al.6 reported 74.5% diagnostic yield of EUS-FNA for the diagnosis of gastric SMT. Patient age of under 60 years old and location of SMT at lower third area of the stomach were the predictive factors of inadequate tissue yield in EUS-FNA. SMT at lower stomach area was difficult to obtain adequate sample.6
Turhan et al.19 performed a prospective study of EUS-FNA for diagnosis of upper gastrointestinal submucosal lesions and reported 82.9%, 73.3%, 87.9%, 64.7%, and 80% for the sensitivity, specificity, positive, and negative predictive values and accuracy of EUS-FNA, respectively, for upper gastrointestinal tract submucosal mesenchymal tumors.
In handling specimens by EUS-FNA in GISTs, it is important to prepare aspirated tissues for cytology and histology in two ways.8 In cases of SMTs, aspirated materials using EUS-FNA were processed for cytology and histology including immunocytochemical and immunohistochemical staining for C-KIT, and 81.6% of the cases were found adequate for cytological and histological examinations. On cytology, cluster types of A (piled clusters with high cellularity showing fascicular pattern) and B (thin layered clusters with high cellularity showing a fascicular pattern) were strongly associated with histologic diagnosis of GIST. Type C (mono-layered clusters or scattered cells) further needed confirming C-KIT positivity and histology.8
To improve diagnostic accuracy for various targets, EUS-guided 19-, 22-, and 25-gauge needles are introduced. Larger needles are expected to improve the diagnostic accuracy of EUS-FNA than smaller needles. Kida et al.20 compared the diagnostic accuracy of EUS-FNA using both 22- and 25-gauge needles in the same patients. Tissue sampling rates and diagnostic accuracy were similar between 22- and 25-gauge needles in every lesion. In 11 SMTs, sampling rates were 100% in both cytology and histology. Twenty-five-gauge needle easily punctured small SMTs and were superior to 22-gauge needle for the small, mobile target lesions.
Some studies were performed to determine how many passes are needed for accurate diagnosis. In SMTs, the accuracy of EUS-FNA increased gradually with each consequent pass to reach a plateau after the 4th pass.21 In a Japanese study, sample adequacy was 83% with 2.5±0.7 passes, which was significantly better for lesions greater than 2 cm.4 With 5.3 needle passes, diagnostic accuracy was 83.9% including diagnostic and suspicious sampling.22
The presence of an on-site cytopathologist in the endoscopy unit and higher number of passes for EUS-FNA are important factors to increase the sensitivity of EUS-FNA.19 EUS-FNA with an on-site cytopathologist can result in 10% to 15% increase in the rate of definite cytologic diagnosis.23
Immediate on-site preliminary diagnosis was possible in 82.35% by smear alone for stomach GISTs using 22-gauge EUS-FNA.14
Important differential diagnoses of the GISTs include epithelial neoplasms or malignant lymphomas. Differential diagnosis of GISTs with other mesenchymal tumors, such as leiomyomas, leiomyosarcomas, or schwannomas, is not possible on routine cytologic examinations, but adding immunocytochemical staining for C-KIT and/or CD34 is helpful for correct diagnosis of GIST.
About 95% of GISTs have a positive reaction for C-KIT on immunohistochemistry, and 78% to 88% have mutations of the c-kit gene.8 Molecular analysis for c-kit mutation can be done on cell block materials from EUS-FNA.5
Fu et al.17 reported diagnostic yield of 10 GISTs by cytology and immunohistochemical staining using EUS-FNA specimens. Diagnostic yield by cytology was 80%, which was confirmed by strong and diffuse tumor cell C-KIT immunoreactivity in the cell blocks. These data suggests that GIST can be diagnosed accurately using EUS by combining cytologic and immunocytochemical studies.
EUS-TCB that provides core tissue specimen can add significant information to EUS-FNA in selective patients. EUS-TCB was useful when immunohistochemical stain was needed in SMTs and lymphomas, for example, to confirm the primary or metastatic origin of mediastinal masses and necrotic tumors. Some difficulties, such as needle stiffness, misfire of the needle inside the lesion and procedural difficulty when the lesion was in the distal antrum, were reported using EUS-TCB.23 The accuracy of dual sampling (EUS-TCB with EUS-FNA) is superior to either technique alone. Sequential sampling (EUS-TCB with EUS-FNA rescue) has similar accuracy to that of dual sampling. EUS-TCB is superior to EUS-FNA in benign tumors or if immunostaining is required. In most instances, EUS-TCB does not offer additional benefit to EUS-FNA, but EUS-TCB should be considered when tissue architectural details and immunostaining are required (Figs. 1-5). Though the likelihood of obtaining adequate tissue was similar between EUS-FNA and EUS-TCB, accuracy for specific diagnosis was higher in EUS-TCB compared to EUS-FNA (68.4% vs. 5.3%, p<0.005).24
To overcome the limitation of EUS-TCB needle, new histology needle with a core trap, a 19 G EUS-FNB device (ProCore; Cook Endoscopy, Winston-Salem, NC, USA), has been developed.21 It was developed with reverse bevel technology to enable the acquisition of core specimens for histologic analysis. In a European study, histologic samples that were obtained with this new 19 G EUS-FNB needle showed diagnostic accuracy of more than 90%.25 The 22 G FNB device for transduodenal approach is also available.26
Ki-67 staining of EUS-FNA specimen is helpful for differentiation of aggressiveness of GISTs.
Ando et al.16 differentiated malignant GISTs from benign GISTs by using mitotic counts and Ki-67 stain on cytologic cell block with over 90% accuracy. Presence of mitoses in specimens by EUS-FNA was associated with malignant GISTs. All the histologically malignant GISTs showed Ki-67 (MIB-1) labelling index >3%.5,9
The MIB-1 labelling indices in specimens of high grade malignancy are significantly higher than those of low grade malignancy. When MIB-1 labelling index >5% was defined as high grade malignancy, the diagnostic accuracy was 85.7%.10
But in some reports, Ki-67 stain in core biopsy specimens does not correlate well with resected specimens.19 Resected gastric GISTs sometimes show intra- or interlobular heterogeneity.19 Every GIST, even small ones, have malignant potential. Cytomorphologic feature alone is insufficient for deciding malignant potential, and special immunohistochemical stains are needed for final diagnosis. Combining EUS-FNA cytologic, histologic findings with immunohistochemical stain can increase the diagnostic yields.
There are some cases of GISTs that are negative for KIT on cell blocks but positive on the resected surgical pathology. DOG1 (discovered on GIST1, anoctamin1, and ANO1) appears to be expressed highly in GISTs based on gene expression profiling.27 This gene encode a calcium activated chloride channel in the interstitial cell of Cajal, which has a critical role in peristalsis. DOG1 was the more sensitive marker than KIT in all GIST cytologic cell blocks.27
EUS-FNA and EUS-TCB have limitation in diagnostic accuracy. Combining these two methods can increase the overall diagnostic accuracy up to 95% (76% for EUS-FNA only and 76% for EUS-TCB only) even without an immediate on-site cytopathologist.23 This finding is hard to apply in practice, however, due to more needle passes and higher costs of EUS-FNA and EUS-TCB. Instead, one method might be used as a rescue strategy when another one is failing.
Applying continuous suction with a syringe during EUS-FNA improves the sensitivity of diagnosis of malignancy in patients with solid masses.21
In SMTs, endoscopic forceps biopsy using bite-on-bite technique can provide specimens adequate for diagnosis, so bite-on-bite biopsy should be the first diagnostic step if available.21
Diagnostic yield of EUS-FNA is moderate and limited by unsatisfactory immunostaining in some patients. It can be improved by obtaining samples for cytopathological plus histopathological examinations. The diagnostic yield of EUS-TCB is similar to that of EUS-FNA. There are some cases that EUS-guided sampling is not likely to impact management, so it is not indicated in patients with the followings: 1) plan of surgery for SMT related symptoms, 2) typical echo features of a lipoma, and 3) small (<2 cm) SMTs of the esophagus and the stomach. Also the clinical benefit of EUS-guided sampling in patients with hypoechoic esophageal or gastric SMTs >2 cm is usually limited and should not be overstated.28
EUS-guided sampling is indicated in the following situations: 1) SMTs with a presumptive diagnosis of unresectable GIST for which treatment with tyrosine kinase inhibitors is contemplated, 2) patient with previous history of malignancy with a SMT that may be consistent with a metastasis, 3) suspected diagnosis of lymphoma, neuroendocrine tumor or extrinsic tumor based on EUS, biological or clinical criteria.28
For duodenal and colorectal SMTs, there are not sufficient data to suggest any recommendation.
Tumor seeding after percutaneous biopsy for malignant GISTs were reported.29 There have been no reports of cancer seeding after EUS-FNA for malignant subepithelial tumors, but peritoneal seeding can be developed if FNA needle penetrates the whole gastric wall and reach the peritoneal side. We should pay more attention, therefore, to make sure the needle does not penetrate the tumor during EUS-FNA procedure.
EUS surveillance of SMT is often used in cases with gastric GISTs smaller than 2 cm, and tumor growth is an indicator for malignancy potential requiring caution. Follow-up at 1-year interval is recommended, and extended follow-up is suggested if the lesion size was unchanged over the past 2 consecutive follow-up EUS.9
Japanese guidelines recommend endoscopic examination once or twice per year for subepithelial lesions less than 2 cm in diameter.30
Guidelines on EUS surveillance of non-resected SMT are required.
For definite diagnosis of submucosal lesions, tissue acquisition from submucosal lesion is necessary. EUS-FNA is a good method for the tissue diagnosis of submucosal lesions. EUS-TCB providing core tissue specimen is useful when immunohistochemical staining and tissue architectural details are required. There are problems of procedural difficulties, however, and a lot of efforts have been made to increase the diagnostic yield of EUS-FNA in submucosal lesions.
For accurate diagnosis of submucosal lesions using EUS-FNA specimen, both cytologic and histologic examinations including immunohistochemical stains are necessary. Recently, EUS-FNB needle, a new histologic needle with core trap, has been developed enabling acquisition of core specimens with promising results. To increase diagnostic accuracy of EUS-FNA, we should pay attention to appropriate EUS-FNA procedure and specimen processing. EUS is more accurate in the diagnosis of submucosal lesions if combined with cytology, histology, and immunohistochemical staining using cell blocks.
References
1. Landi B, Palazzo L. The role of endosonography in submucosal tumours. Best Pract Res Clin Gastroenterol. 2009; 23:679–701. PMID: 19744633.

2. Papanikolaou IS, Triantafyllou K, Kourikou A, Rösch T. Endoscopic ultrasonography for gastric submucosal lesions. World J Gastrointest Endosc. 2011; 3:86–94. PMID: 21772939.

3. Ji JS, Lee BI, Choi KY, et al. Diagnostic yield of tissue sampling using a bite-on-bite technique for incidental subepithelial lesions. Korean J Intern Med. 2009; 24:101–105. PMID: 19543487.

4. Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010; 71:913–919. PMID: 20226456.

5. Zhang S, Defrias DV, Alasadi R, Nayar R. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA): experience of an academic centre in the USA. Cytopathology. 2010; 21:35–43. PMID: 19843142.

6. Suzuki T, Arai M, Matsumura T, et al. Factors associated with inadequate tissue yield in EUS-FNA for gastric SMT. ISRN Gastroenterol. 2011; 2011:619128. PMID: 21991522.

7. Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992; 38:172–173. PMID: 1568614.

8. Yoshida S, Yamashita K, Yokozawa M, et al. Diagnostic findings of ultrasound-guided fine-needle aspiration cytology for gastrointestinal stromal tumors: proposal of a combined cytology with newly defined features and histology diagnosis. Pathol Int. 2009; 59:712–719. PMID: 19788616.

9. Sakamoto H, Kitano M, Kudo M. Diagnosis of subepithelial tumors in the upper gastrointestinal tract by endoscopic ultrasonography. World J Radiol. 2010; 2:289–297. PMID: 21160683.

10. Okubo K, Yamao K, Nakamura T, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of gastrointestinal stromal tumors in the stomach. J Gastroenterol. 2004; 39:747–753. PMID: 15338368.

11. Rösch T, Kapfer B, Will U, et al. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002; 37:856–862. PMID: 12190103.
12. Sato T, Peiper M, Fritscher-Ravens A, Gocht A, Soehendra N, Knoefel WT. Strategy of treatment of submucosal gastric tumors. Eur J Med Res. 2005; 10:292–295. PMID: 16055400.
13. Mazur MT, Clark HB. Gastric stromal tumors: reappraisal of histogenesis. Am J Surg Pathol. 1983; 7:507–519. PMID: 6625048.

14. Chatzipantelis P, Salla C, Karoumpalis I, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy in the diagnosis of gastrointestinal stromal tumors of the stomach: a study of 17 cases. J Gastrointestin Liver Dis. 2008; 17:15–20. PMID: 18392238.
15. Hunt GC, Rader AE, Faigel DO. A comparison of EUS features between CD-117 positive GI stromal tumors and CD-117 negative GI spindle cell tumors. Gastrointest Endosc. 2003; 57:469–474. PMID: 12665755.

16. Ando N, Goto H, Niwa Y, et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002; 55:37–43. PMID: 11756912.

17. Fu K, Eloubeidi MA, Jhala NC, Jhala D, Chhieng DC, Eltoum IE. Diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration biopsy: a potential pitfall. Ann Diagn Pathol. 2002; 6:294–301. PMID: 12376922.
18. Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007; 13:2077–2082. PMID: 17465451.

19. Turhan N, Aydog G, Ozin Y, Cicek B, Kurt M, Oguz D. Endoscopic ultrasonography-guided fine-needle aspiration for diagnosing upper gastrointestinal submucosal lesions: a prospective study of 50 cases. Diagn Cytopathol. 2011; 39:808–817. PMID: 20836005.

20. Kida M, Araki M, Miyazawa S, et al. Comparison of diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration with 22- and 25-gauge needles in the same patients. J Interv Gastroenterol. 2011; 1:102–107. PMID: 22163079.
21. Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) technical guideline. Endoscopy. 2012; 44:190–206. PMID: 22180307.

22. Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009; 69:1218–1223. PMID: 19394006.

23. Storch I, Jorda M, Thurer R, et al. Advantage of EUS Trucut biopsy combined with fine-needle aspiration without immediate on-site cytopathologic examination. Gastrointest Endosc. 2006; 64:505–511. PMID: 16996340.

24. Săftoiu A, Vilmann P, Guldhammer Skov B, Georgescu CV. Endoscopic ultrasound (EUS)-guided Trucut biopsy adds significant information to EUS-guided fine-needle aspiration in selected patients: a prospective study. Scand J Gastroenterol. 2007; 42:117–125. PMID: 17190771.

25. Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011; 73:1189–1196. PMID: 21420083.

26. Varadarajulu S, Fockens P, Hawes RH. Best practices in endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. Epub 2012 Apr 1. DOI: http://dx.doi.org/10.1016/j.cgh.2012.03.017.

27. Hwang DG, Qian X, Hornick JL. DOG1 antibody is a highly sensitive and specific marker for gastrointestinal stromal tumors in cytology cell blocks. Am J Clin Pathol. 2011; 135:448–453. PMID: 21350101.

28. Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2011; 43:897–912. PMID: 21842456.

29. Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000; 51:184–190. PMID: 10650262.

30. Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008; 13:416–430. PMID: 18946752.

Fig. 1
Endoscopic finding. An extrinsic compression and erosions of salt and pepper type were found at the anterior wall of the duodenal bulb.

Fig. 2
Computed tomographic finding. The enhanced computed tomography scan showed a large mass, proving heterogenous enhancement in the periphery and low attenuation, suggesting necrosis, in the central area of the lesion.
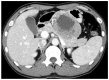
Fig. 3
Endoscopic ultrasonography (EUS) Findings. (A) A 78×86 mm hypoechoic mass was found, characterizing anechoic in the center of the lesion. (B) EUS-guided Trucut biopsy was performed.
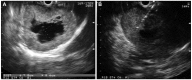
Fig. 4
Gross findings. (A) It showed malignant gastrointestinal stromal tumor (GIST) (>10 cm), subserosal, exophytic polypoid type at the stomach body along the lesser curvature. (B) Cut section showed malignant GIST (>10 cm) with solid and cystic, hemorrhagic and necrotic, fish-flesh, sarcomatous cut surfaces.

Fig. 5
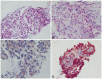
Microscopic findings. (A) Cellular epithelioid malignant gastrointestinal stromal tumor (GIST) with discohesive pattern of growth, nuclear anaplasia & pleomorphism (H&E stain, ×100). (B) Cellular epithelioid malignant GIST with frequent mitoses >5/50 high power fields (H&E stain, ×200). (C) Cytoplasmic membranous immunoreactivity for C-KIT (CD117) (Immunohistochemical stain, ×200). (D) Diffuse strong cytoplasmic immunoreactivity for CD34 (Immunohistochemical stain, ×40).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download