Abstract
Background/Aims
Early gastric cancer (EGC) that is undifferentiated or shows submucosal invasion has not been generally accepted as an indication for endoscopic treatment. But recently, experiences with endoscopic submucosal dissection (ESD) for undifferentiated EGC or submucosal invasive (SM) EGC have increased. The aim of this study was to evaluate clinical outcomes of ESD for EGC with undifferentiation or submucosal invasion.
Methods
Between August 2005 and August 2009, among 210 EGCs treated using ESD at our hospital, 18 lesions were diagnosed as undifferentiated gastric cancer and 41 as SM gastric cancer. A retrospective analysis was done on the medical records of these patients.
Results
Mean follow-up periods were 19.39±11.2 months. During the follow-up period, local recurrence was noted in 4 lesions. Local recurrence rates of the EGC groups (group 1, mucosal cancer with undifferentiation; group 2, SM cancer with differentiation; group 3, SM cancer with undifferentiation) were 10%, 4.5%, and 50%, respectively. Groups 1 and 2 were not significantly different in local recurrence rates compared to the mucosal cancer with differentiation group (p=0.061, p=0.125, respectively). The undifferentiated EGC group was significantly lower in curability using ESD than the differentiated EGC group (55.6% vs. 89.6%, p=0.000). The curability of the SM EGC group was lower than the mucosal EGC group (36.6% vs. 98.9%).
Conclusions
Complete resection using ESD is difficult in undifferentiated and SM gastric cancers. SM cancer with undifferentiation should be treated immediately by salvage operation. For mucosal cancer with undifferentiation or SM cancer with differentiation, one should consider careful short-term follow-up.
Go to : 
Gastrectomy with lymph node (LN) dissection was considered as the only standard treatment of early gastric cancer (EGC) in the past, but a curative endoscopic treatment is widely used recently. Endoscopic treatment of EGC is less invasive than surgery, reduces medical costs, improves quality of life of patients, and is as effective as surgery. Endoscopic treatment for EGC is performed more frequently with the increased detection of EGC by universal health check-ups. But curative endoscopic mucosal resection of EGC was performed only in selected cases depending on the differentiation, size and depth of invasion of their lesions. Traditional indication of endoscopic treatment was an elevated type cancer of less than 2 cm in size (EGC I or IIa) and a depressed type of less than 1 cm without ulcer (EGC IIc) among differentiated EGCs confined to the mucosa.1 This was to minimize the possibility of LN metastasis and local recurrence. With the recent development of endoscopic maneuver and the accessory tools, the introduction of endoscopic submucosal dissection (ESD) enabled endoscopic treatment of lesions deemed untreatable in the past. ESD improved en bloc resection rate and complete resection rate regardless of the lesions location and size, compared to the conventional endoscopic mucosal resection. ESD also enabled dissection of submucosal layer as deep as 1,500 µm and extended the indication of endoscopic treatment regardless of the size, location, and shape of lesions, which were limitations of the conventional endoscopic treatment.2 ESD still cannot be performed confidently in undifferentiated or submucosal invasive (SM) EGC, though. Such lesions favor surgery than endoscopic treatment due to the increased frequency of local recurrence, micro-invasion, and LN metastasis.3,4
But accurate assessment of depth of invasion is difficult in pre-ESD lesion. When using endoscopic ultrasonography (EUS) and conventional endoscopy together, accuracy of invasion depth is about 90% on the lesion confined to mucosa only, about 70-80% on deeper lesions, particularly SM 1 lesion is very difficult to detect due to its depth is slight.5 There might be also a difference between the endoscopic biopsy result before the endoscopic treatment and the histologic findings after the treatment, such as that a differentiated EGC before the endoscopic treatment was often confirmed as undifferentiated after the treatment.6,7 Such pathologic findings after the treatment requires decision about additional treatment or surgery, but currently there is no definite criteria for such occasions.
This study was performed to investigate the clinical course of cases confirmed as undifferentiated or SM EGC according to the pathologic findings after ESD. Local recurrent cases were analyzed to determine the cases who need additional treatment or surgery and who are available for follow-up.
Go to : 
Among 210 cases of ESD due to EGC at our hospital between August 2005 and August 2009, 41 cases were SM lesions and 18 cases were undifferentiated lesions according to the pathologic reports after the ESD. A retrospective study was performed in these cases based on their medical records.
The indication of ESD included well or moderate differentiation from biopsy, mucosal cancer (M cancer) from endoscopic gross findings, and cases without prominent ulcer on the surface of the lesion. Patients rejecting the surgery or with unsuitable condition for surgery also received ESD even though the lesion is undifferentiated or invading SM1 from endoscopic gross finding. Pathology of pre-ESD was checked from the biopsy reports of our hospital or other hospitals. Abdominal computed tomography (CT) was performed in every patient to confirm the absence of distant metastasis or local LN metastasis.
En bloc resection was attempted whenever possible, and the resected surfaces were sliced at 2 mm intervals to confirm the complete resection, which was defined by no histological tumor cell in the margin and no tumor emboli in lymphovascular space. Rates of en bloc resection and complete resection were analyzed. Submucosal layer of the resected tissue was divided in 3 equal parts and an invasion less than 500 µm was classified as SM1, more than 500 µm as SM2, or as SM3.8 EGC was classified as differentiated adenocarcinoma when the lesion was well differentiated or moderately differentiated, or as undifferentiated when the lesion was poorly differentiated or a signet ring cell cancer.
Follow-up endoscopy and biopsy was performed at 3, 6, and 12 months after the treatment, and annual follow-up endoscopy and abdomen CT was performed thereafter if not showing other abnormality. The period of follow-up endoscopy was adjusted in some cases based on the clinician's decision. Additional biopsy was performed to confirm the presence of residual lesion when histologic tumor cell was found at the margin of the resected lesion. So, the follow-up was continued as planned when there was no residual lesion, but the surgery was performed when a residual lesion was found.
SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Chi-square test was used for univariate analysis of factors associated with local recurrence and the comparison of differentiated and undifferentiated cancer, and mucosal and SM cancer. Logistic regression analysis was performed for multivariate analysis of factors associated with local recurrence. p-value less than 0.05 was determined statistically significant.
Go to : 
Pathologic result after ESD was confirmed as differentiated cancer in 192 lesions (91.4%) and undifferentiated cancer in 18 lesions (8.6%). Among 18 lesions of undifferentiated cancer, 8 lesions were determined undifferentiated, 9 lesions differentiated, and 1 lesion adenoma by pre-ESD pathology (Fig. 1). One hundred sixty-nine lesions (80.5%) were localized to the mucosa, 35 lesions (16.7%) were SM1 invasion, and 6 lesions (2.8%) were SM2 invasion. En bloc resection rate was 80.5% (169 lesions) and the complete resection rate was 86.2% (181 lesions) (Table 1).
EGC was differentiated in 192 lesions and undifferentiated in 18 lesions. Undifferentiated cancer had larger tumor size and significantly more submucosal invasion and lymphovascular tumor emboli (LVTE) than differentiated cancer. Positive rate of resected margin was significantly higher in undifferentiated cancer, leading to a significantly lower complete resection rate (10/18, 55.6%). Among 8 lesions with incomplete resection, 4 lesions were positive vertical margin, 1 lesion positive horizontal margin, and 1 lesion positive both vertical and horizontal margin. Two lesions had positive LVTE. There was no difference between the en bloc resection rates of the two groups (Table 2).
One hundred sixty-nine lesions were determined as M cancer and 41 lesions were submucosal invasion. EGC with submucosal invasion had larger tumor size and significantly more undifferentiated and LVTE positive than M cancer. Positive rate of resection margin was significantly higher in EGC with submucosal invasion, leading to a lower complete resection rate (36.6%) compared to M cancer (98.8%). Among 26 lesions with incomplete resection, 14 lesions were positive vertical margin, 2 lesions were positive horizontal margin, and 10 lesions were positive LVTE. There was also no significant difference between the en bloc resection rates of the two groups (Table 3).
The mean follow-up period among 191 patients who were available for follow-up was 19.39±11.2 months. No regional LN enlargement or distant metastasis suggesting recurrence was found at follow-up abdomen CT. Local recurrence was confirmed in 4 lesions (2%) by endoscopic biopsy during follow-up. One case (0.6%) had recurrence among 164 cases with M cancer available for the follow-up.
One case (4.1%) out of 24 cases with SM1 invasive cancer were also recurred. There was a statistically significant difference in the recurrence rates between M cancer and SM1 invasive cancer (p=0.001). Three cases that were SM2 invasion but the patients rejected the surgery were followed, and 2 cases (66.6%) among them had recurrence. There was also statistically significant difference in the recurrence rates between SM1 and SM2 invasive cancer (p=0.025). One case (0.5%) among 177 cases of differentiated cancer and 3 cases (21.4%) among 14 cases of undifferentiated cancer had recurrence, with statistically significant difference between the two (p=0.001) (Table 4).
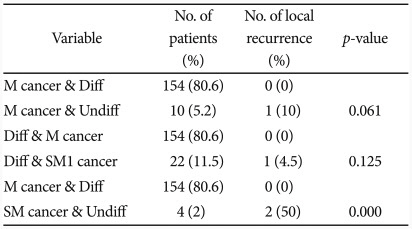
When both differentiation and depth of invasion were considered, 154 cases (80.6%) with differentiated M cancer did not experience recurrence, while 1 case (10%) among 10 (5.2%) undifferentiated M cancer cases had recurrence, and there was no statistically significant difference between the two groups (p=0.061). Among 22 cases (11.5%) of differentiated SM1 cancer, 1 case (4.5%) experienced recurrence, without significant difference with the differentiated M cancer group (p=0.125). But 2 case (50%) among 4 cases of undifferentiated SM cancer were recurred with significant difference with the differentiated M cancer group (p=0.000) (Table 5).
Local recurrence was confirmed at 4 lesions during follow-up after ESD; 2 lesions were undifferentiated EGC, and 3 lesions were SM cancer. One lesion was undifferentiated EGC confined to the mucosa, which was completely resected but recurred at 31 months after the ESD. Table 6 shows the additional treatments applied and pathologic stages after the surgery of these cases.
When the recurrence rate was subdivided by the depth of invasion, the recurrence rate was 0.6% (1/164) for EGC confined to the mucosa, 4.1% (1/24) for SM1, and 66.6% (2/3) for SM2, indicating an increasing pattern with deeper invasion. The recurrence rate of undifferentiated cancer was 21.4% (3/14), significantly higher than 0.5% (1/177) in differentiated cancer. Positivity of resection margin and the size of the lesion also affected the local recurrence, but only the differentiation was an independent factor at multivariate analysis, significantly affecting the local recurrence (Table 7).
Go to : 
Undifferentiated or SM EGC is still limited from endoscopic treatment, despite recent development of ESD, and only carefully discussed based on a few studies.9,10 Assessment of LN metastasis before endoscopic resection is important for endoscopic treatment of EGC, which was reported as 0.4% in differentiated M cancer and 4.2% in undifferentiated M cancer.11 This difference becomes an important criteria when selecting a candidate patient for mucosal resection. In addition to the high LN metastasis rate, undifferentiated EGC is more likely to invade submucosal layer even in small size and distributed in subepithelial lateral spreading or discontinuous pattern, which meant that an endoscopic treatment was not easily applicable due to the difficulty of defining definite lesion area before the ESD.11-13 EGC with submucosal invasion was also excluded from the indication of ESD because of high (10-25%) frequency of LN metastasis.14,15 Undifferentiated or SM EGC were candidates of surgery rather than endoscopic treatment, for these reasons.
The existing indication on endoscopic resection of EGC is based on surgical data, which were more focused on the probability of LN metastasis. These indications, however, did not consider actual problems associated with endoscopic procedures, such as incomplete resection, which could be more important problems particularly in undifferentiated cancer or SM cancer.9 This study has the meaning in that undifferentiated or SM cancer after ESD were investigated.
The complete resection rate 55.6% among 18 cases confirmed as undifferentiated cancer after ESD was significantly lower than 89.1% in differentiated cancer. This result was comparable with other studies reporting clinical outcomes of ESD in undifferentiated cancer.16 It is possible, as mentioned earlier, that the actual size and depth of the lesion was larger than expected before ESD due to the characteristics of undifferentiated cancer.16 The complete resection rate (36.6%) of 41 cases of SM cancer was also lower than that of M cancer (98.8%), reflecting the fact that complete resection of SM cancer is more difficult than M cancer. These actual problems associated with procedure, such as incomplete resection, are other reasons why ESD is difficult in undifferentiated or SM EGC.
Local recurrence was confirmed in 4 cases (2%) at follow-up endoscopic biopsy among 191 patients available for follow-up after ESD. Another study on ESD for EGC reported 3.7% for recurrence rate.17 When these cases were analyzed based on their depth of invasion and differentiation, the recurrence rate was higher when the lesion was deeper and undifferentiated, probably because the complete resection rate is decreased in such cases, as mentioned earlier. This study reported relatively higher recurrence rate (21.4%) after ESD for undifferentiated cancer compared to that reported by another study (5.1%), probably because 2 out of 3 recurrent cases were resected incompletely.9
Like this, ESD is difficult to apply in SM or undifferentiated EGC. The important problem is that the discrepancy of differentiation before and after the ESD frequently occurs and there is the difficulty of predicting the depth of tumor invasion accurately. Even the EUS using to evaluate the depth of invasion accurately before ESD shows similar accuracy as endoscopic gross findings. Only 90% of accuracy in EUS produced by combined with endoscopic gross finding in M cancer.5 Also, a previous study reported 23.7% of discrepancy in differentiation,6 and only 50% among undifferentiated patients before ESD were diagnosed as undifferentiated cancer again after ESD in this study. This discrepancy in the histological diagnosis may be explained by the fact that endoscopic biopsy is collected from only a part of lesion and the histologic type is determined by the dominant type among multiple histologic types.6
When the pathologic result after ESD diverged from the conventional indication, additional procedure or salvage operation should be considered. Some studies reported the possibility of endoscopic treatment of undifferentiated cancer, and even the possibility of endoscopic treatment of EGC with SM1 invasion. It is suggested, therefore, that surgery is not always the best choice for cases not indicated for endoscopic treatment, considering the increased risk of surgery with comorbid diseases and the patient's quality of life after the surgery. We evaluated the cases that were available to follow-up after ESD and cases that had to consider salvage operation early with respect to their local recurrence. Compared to the case with differentiated EGC confined to the mucosa that did not have local recurrence, cases with undifferentiated EGC confined to the mucosa and cases with differentiated EGC with SM1 invasion did not show statistically significant difference in their local recurrence rates. The cases with undifferentiated EGC with SM invasion showed recurrence rate as high as 50%. This result suggests that cases of undifferentiated EGC with SM invasion requires early salvage operation, while cases of undifferentiated EGC confined to the mucosa or differentiated EGC with SM1 invasion could be better to wait and see with short-term follow-up rather than to perform salvage operation immediately. Although there is a risk of missing the opportunity of complete cure, careful short-term follow-up could be possible for well-selected patients, considering the increased risk of surgery with comorbid diseases and the patient's quality of life after the surgery. Additionally, among 11 patients who received early salvage operation after ESD in this study, only 3 patients had residual tumor, 8 patients did not have any residual tumor, and regional LN metastasis was detected only in 1 patient. This result is similar to the positive rate of residual tumor (32.5%) reported from other studies that performed surgery after endoscopic resection.18
In addition to the depth of invasion and differentiation, size of tumor and positive tumor cell of resection margin were also factors associated with local recurrence of post-ESD lesion. Multivariate analysis revealed differentiation was the only independent factor affecting local recurrence. Other studies reported positive tumor cell of resection margin, the depth of invasion, or the size of tumor as independent factors affecting the local recurrence.16,17,19 It seems the small size of our study was the reason of different result with other studies. This study has several limitations including that the assessment of LN metastasis was evaluated only by radiologic findings except for patients who received surgery, the follow-up period was not long enough, the number of enrolled patients were small, and designed as a retrospective study.
In conclusion, undifferentiated or with SM invasion EGC shows low complete resection rate and high local recurrence rate after ESD. When the EGC was diagnosed as undifferentiated or SM invasion after ESD, it is required to decide whether or not perform immediate salvage operation, but a short-term follow-up could be possible if the lesion was completely resected and reported as undifferentiated EGC confined to the mucosa or differentiated EGC confined to the SM1. This needs further studies in larger patient groups. Immediate salvage operation is more advisable, however, for undifferentiated EGC with SM invasion.
Go to : 
References
1. Tada M, Murakami A, Karita M, Yanai H, Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993; 25:445–450. PMID: 8261986.

2. Kim DH, Jung HY. Expansion of indication for endoscopic SD in early gastric cancer. J Gastric Cancer. 2010; 10:49–54.

3. Cai J, Ikeguchi M, Maeta M, Kaibara N. Micrometastasis in lymph nodes and microinvasion of the muscularis propria in primary lesions of submucosal gastric cancer. Surgery. 2000; 127:32–39. PMID: 10660756.

4. Cai J, Ikeguchi M, Tsujitani S, Maeta M, Kaibara N. Micrometastasis in lymph nodes of mucosal gastric cancer. Gastric Cancer. 2000; 3:91–96. PMID: 11984717.

5. Yanai H, Matsumoto Y, Harada T, et al. Endoscopic ultrasonography and endoscopy for staging depth of invasion in early gastric cancer: a pilot study. Gastrointest Endosc. 1997; 46:212–216. PMID: 9378206.

6. Park EH, Kang KT, Kim BH, et al. The histologic discrepancy before and after endoscopic submucosal dissection of gastric adenoma and early gastric cancer. Korean J Gastrointest Endosc. 2007; 34:125–131.
7. Kim KM, Park CK. Pathology of endoscopic submucosal dissection: how do we interpret? Korean J Gastroenterol. 2010; 56:214–219. PMID: 20962556.

8. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003; 58(6 Suppl):S3–S43. PMID: 14652541.
9. Kim JH, Lee YC, Kim H, et al. Endoscopic resection for undifferentiated early gastric cancer. Gastrointest Endosc. 2009; 69:e1–e9. PMID: 19327466.
10. Park DJ, Lee HK, Lee HJ, et al. Lymph node metastasis in early gastric cancer with submucosal invasion: feasibility of minimally invasive surgery. World J Gastroenterol. 2004; 10:3549–3552. PMID: 15534904.

11. Abe N, Watanabe T, Sugiyama M, et al. Endoscopic treatment or surgery for undifferentiated early gastric cancer? Am J Surg. 2004; 188:181–184. PMID: 15249247.

12. Nasu J, Nishina T, Hirasaki S, et al. Predictive factors of lymph node metastasis in patients with undifferentiated early gastric cancers. J Clin Gastroenterol. 2006; 40:412–415. PMID: 16721222.

13. Park YD, Chung YJ, Chung HY, et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy. 2008; 40:7–10. PMID: 18210339.

14. Namieno T, Koito K, Higashi T, Sato N, Uchino J. General pattern of lymph node metastasis in early gastric carcinoma. World J Surg. 1996; 20:996–1000. PMID: 8798355.
15. Yasuda K, Shiraishi N, Suematsu T, Yamaguchi K, Adachi Y, Kitano S. Rate of detection of lymph node metastasis is correlated with the depth of submucosal invasion in early stage gastric carcinoma. Cancer. 1999; 85:2119–2123. PMID: 10326688.

16. Kang HY, Kim SG, Kim JS, Jung HC, Song IS. Clinical outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Surg Endosc. 2010; 24:509–516. PMID: 19585066.

17. Park JC, Lee SK, Seo JH, et al. Predictive factors for local recurrence after endoscopic resection for early gastric cancer: long-term clinical outcome in a single-center experience. Surg Endosc. 2010; 24:2842–2849. PMID: 20428894.

18. Bae JM, Kim SW, Kim SW, Song SK. Clinicopathological characteristics of patients who received additional gastrectomy after endoscopic resection due to gastric cancer. J Korean Surg Soc. 2010; 78:87–92.

19. Takenaka R, Kawahara Y, Okada H, et al. Risk factors associated with local recurrence of early gastric cancers after endoscopic submucosal dissection. Gastrointest Endosc. 2008; 68:887–894. PMID: 18565523.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print









 XML Download
XML Download