1. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999; 341:1725–1730. PMID:
10580071.

2. Kiberd BA, Rose C, Gill JS. Cancer mortality in kidney transplantation. Am J Transplant. 2009; 9:1868–1875. PMID:
19563337.

3. Jackson-Spence F, Gillott H, Tahir S, Nath J, Mytton J, Evison F, et al. Mortality risk after cancer diagnosis in kidney transplant recipients: the limitations of analyzing hospital administration data alone. Cancer Med. 2018; 7:931–939. PMID:
29441723.

4. Opelz G, Naujokat C, Daniel V, Terness P, Döhler B. Disassociation between risk of graft loss and risk of non-Hodgkin lymphoma with induction agents in renal transplant recipients. Transplantation. 2006; 81:1227–1233. PMID:
16699447.

5. Kwon H, Kim YH, Choi JY, Sung S, Jung JH, Park SK, et al. Analysis of 4000 kidney transplantations in a single center: across immunological barriers. Medicine (Baltimore). 2016; 95:e4249. PMID:
27512839.
6. Echterdiek F, Latus J, Schwenger V. Immunosuppression in sensitized recipients. Curr Opin Organ Transplant. 2020; 25:80–85. PMID:
31815787.

7. Speer C, Kälble F, Nusshag C, Pego da Silva L, Schaier M, Becker LE, et al. Outcomes and complications following ABO-incompatible kidney transplantation performed after desensitization by semi-selective immunoadsorption: a retrospective study. Transpl Int. 2019; 32:1286–1296. PMID:
31322786.
8. Kim JM, Park H, Jang HR, Park JB, Kwon CH, Huh W, et al. High pretransplant HBV level predicts HBV reactivation after kidney transplantation in HBV infected recipients. Ann Surg Treat Res. 2014; 86:256–263. PMID:
24851227.

9. Genberg H, Hansson A, Wernerson A, Wennberg L, Tydén G. Pharmacodynamics of rituximab in kidney transplantation. Transplantation. 2007; 84(12 Suppl):S33–S36. PMID:
18162986.

10. Mata M, Gerken C, Nguyen P, Krenciute G, Spencer DM, Gottschalk S. Inducible activation of MyD88 and CD40 in CAR T cells results in controllable and potent antitumor activity in preclinical solid tumor models. Cancer Discov. 2017; 7:1306–1319. PMID:
28801306.

11. Bachelet T, Visentin J, Davis P, Taton B, Guidicelli G, Kaminski H, et al. The incidence of post-transplant malignancies in kidney transplant recipients treated with Rituximab. Clin Transplant. 2021; 35:e14171. PMID:
33247459.

12. Yang CY, Lee CY, Yeh CC, Tsai MK. Renal transplantation across the donor-specific antibody barrier: graft outcome and cancer risk after desensitization therapy. J Formos Med Assoc. 2016; 115:426–433. PMID:
26725772.

13. Schrezenmeier E, Budde K, Staeck O, Lehner L, Duerr M, Khadzhynov D, et al. Incidence of infectious disease and malignancies after rituximab therapy in kidney transplant recipients: results from a cohort in Germany. Transplant Proc. 2017; 49:2269–2273. PMID:
29198659.

14. Kamar N, Milioto O, Puissant-Lubrano B, Esposito L, Pierre MC, Mohamed AO, et al. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant. 2010; 10:89–98. PMID:
19656128.

15. Kim YH, Kim JY, Kim DH, Ko Y, Choi JY, Shin S, et al. Pneumocystis pneumonia occurrence and prophylaxis duration in kidney transplant recipients according to perioperative treatment with rituximab. BMC Nephrol. 2020; 21:93. PMID:
32160881.

16. Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004; 4:905–913. PMID:
15147424.

17. Kanaan N, Raggi C, Goffin E, De Meyer M, Mourad M, Jadoul M, et al. Outcome of hepatitis B and C virus-associated hepatocellular carcinoma occurring after renal transplantation. J Viral Hepat. 2017; 24:430–435. PMID:
27917563.

18. Yun EH, Hong S, Her EY, Park B, Suh M, Choi KS, et al. Trends in participation rates of the National Cancer Screening Program among cancer survivors in Korea. Cancers (Basel). 2020; 13:81.

19. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009; 9 Suppl 3:S1–S155.
20. Pedotti P, Cardillo M, Rossini G, Arcuri V, Boschiero L, Caldara R, et al. Incidence of cancer after kidney transplant: results from the North Italy transplant program. Transplantation. 2003; 76:1448–1451. PMID:
14657684.

21. Bustami RT, Ojo AO, Wolfe RA, Merion RM, Bennett WM, McDiarmid SV, et al. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant. 2004; 4:87–93. PMID:
14678038.

22. Tarella C, Passera R, Magni M, Benedetti F, Rossi A, Gueli A, et al. Risk factors for the development of secondary malignancy after high-dose chemotherapy and autograft, with or without rituximab: a 20-year retrospective follow-up study in patients with lymphoma. J Clin Oncol. 2011; 29:814–824. PMID:
21189387.

23. Lee J, Lee JG, Kim S, Song SH, Kim BS, Kim HO, et al. The effect of rituximab dose on infectious complications in ABO-incompatible kidney transplantation. Nephrol Dial Transplant. 2016; 31:1013–1021. PMID:
27190360.

24. Morath C, Mueller M, Goldschmidt H, Schwenger V, Opelz G, Zeier M. Malignancy in renal transplantation. J Am Soc Nephrol. 2004; 15:1582–1588. PMID:
15153569.
25. Lee J, Park JY, Kim DG, Lee JY, Kim BS, Kim MS, et al. Effects of rituximab dose on hepatitis B reactivation in patients with resolved infection undergoing immunologic incompatible kidney transplantation. Sci Rep. 2018; 8:15629. PMID:
30353021.

26. Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015; 62:956–967. PMID:
25595883.

27. Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018; 154:1706–1718. PMID:
29425931.

28. Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, et al. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol. 2017; 3:456–463. PMID:
27657493.

29. Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography: a randomised study. Aliment Pharmacol Ther. 2013; 38:303–312. PMID:
23750991.
30. Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009; 30:37–47. PMID:
19392863.





 PDF
PDF Citation
Citation Print
Print



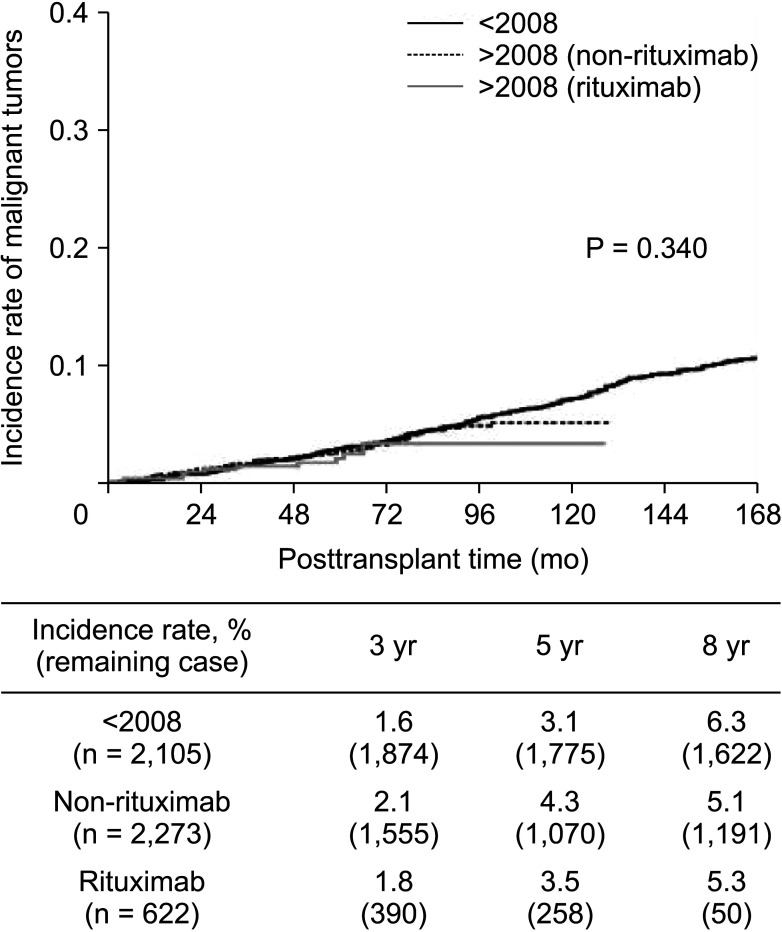
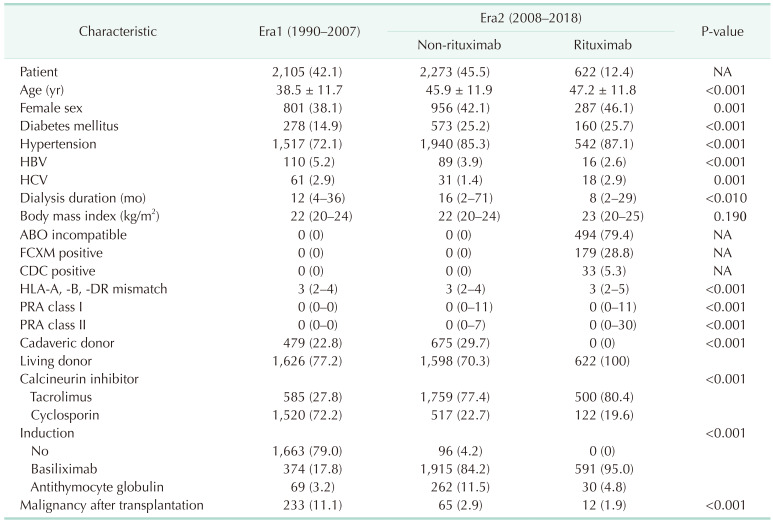
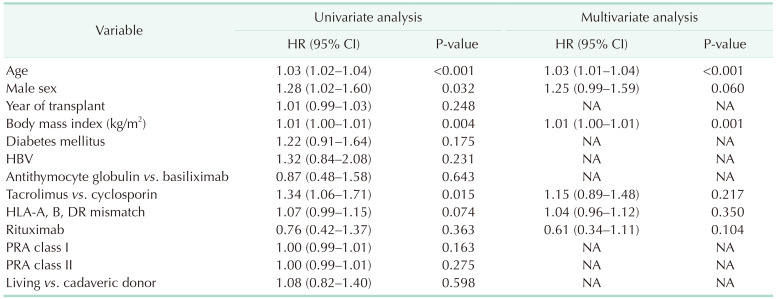
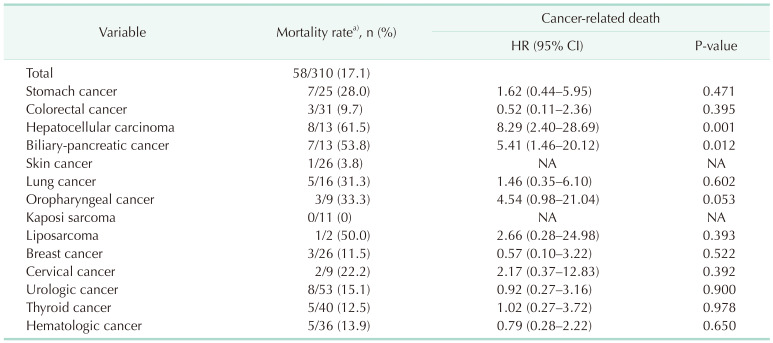
 XML Download
XML Download