INTRODUCTION
Exocrine pancreatic insufficiency (EPI) is a condition caused by reduced or inappropriate secretion or activity of digestive enzymes such as pancreatic juice and pancreatic lipase [
1]. There are various causes of EPI, including chronic pancreatitis, previous history of acute pancreatitis, and pancreatic malignancy [
23]. In particular, pancreatic surgery causes EPI, which causes a sudden impairment in the quality of life, along with malnutrition [
4]. Therefore, the symptoms of EPI caused by pancreatic surgery may be more severe than those of EPI from other causes. However, most studies so far have dealt with EPI after chronic pancreatitis, and few have reviewed EPI after pancreatectomy.
EPI after pancreatic resection should be evaluated from the perspective of short- and long-term outcomes; several methods are available for this evaluation. The methods used to detect EPI are divided into direct and indirect methods. Stool elastase (SE) is one of the most easily available indirect methods for the detection of EPI used in clinical settings. SE employs an enzyme-linked immunosorbent assay (ELISA) technique using monoclonal and polyclonal antibodies. The enzyme concentration in the feces is 5 times higher than that in the pancreatic juice [
56]. In patients with an SE level less than 200 µg/g, EPI is considered. A cutoff value of less than 100 µg/g and 100–200 µg/g were further defined as severe and moderate EPI, respectively. The sensitivity and specificity of SE for detecting EPI by this method is more than 90% [
7].
Among pancreatic resections, EPI is more prevalent in head resection than in tail resection. Even though there are differences depending on the centers, it has been reported that EPI occurs in 70%–80% of pancreatoduodenectomy (PD) or pylorus-preserving PD (PPPD) cases, and 25%–50% of distal pancreatectomy cases [
8910]. In order to study the risk factors for EPI and the serial changes after surgery, the type of surgery should be considered. In addition, to determine how much EPI affects the patient’s nutritional status, a comprehensive approach, such as the evaluation of serial SE level, nutritional parameters, and weight, is required.
Therefore, this study aimed to identify the frequency of EPI incidence after PD and to identify the risk factors for deteriorated exocrine function by measuring patients’ SE levels. In addition, we analyzed the serial changes in nutritional markers and weight according to the SE level.
Go to :

METHODS
Patients
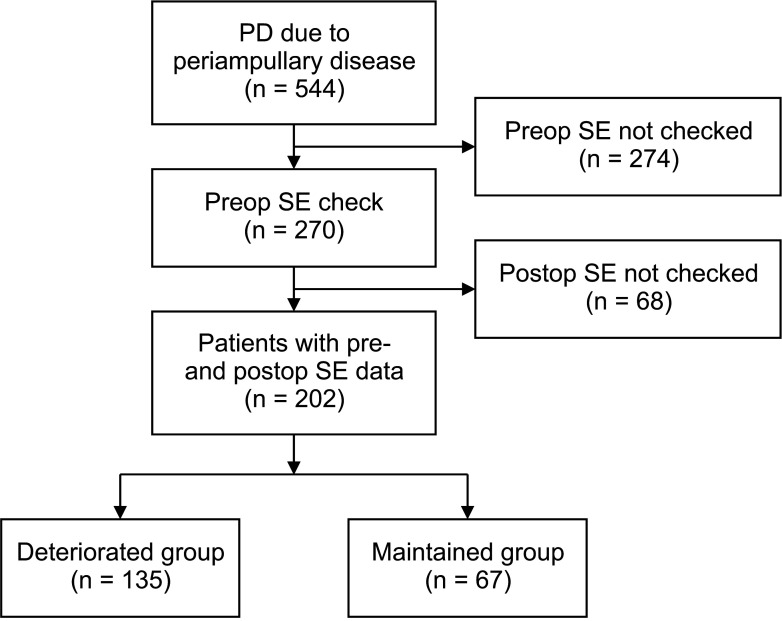
Among patients who underwent PD for periampullary diseases at Seoul National University Hospital (SNUH) from October 2007 through February 2013, patients whose preoperative and postoperative SE levels were evaluated were enrolled in this study. Patients for whom pre- or postoperative SE levels were not checked were excluded. This study was approved by the Institutional Review Board of SNUH (No. 2103-072-1204). It was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
Data collection
Data were prospectively collected from patients who underwent PD. Patients’ medical records, including characteristics, preoperative factors, operative factors, and postoperative factors were reviewed from their electronic medical charts. Patient characteristics included age, sex, preoperative weight, and comorbidities. Preoperative factors included preoperative hemoglobin A1c level, SE level, and tumor origin.
The operative factor included the type of operation. Postoperative factors included tumor pathology and stage of the disease. Factors related to clinical outcomes included the presence of a clinically relevant postoperative pancreatic fistula (CR-POPF), days of postoperative stay, presence of complications, postoperative SE level, and mortality. Postoperative treatments included adjuvant chemotherapy, adjuvant radiotherapy, and pancreatic enzyme replacement therapy (PERT). Patients with all pancreatic cancer and bile duct cancer more than stage II were recommended to have adjuvant chemotherapy. Patients with extensive lymph node metastasis and tumor close to resection margin were recommended to have adjuvant radiotherapy. The final decision of adjuvant chemotherapy or radiotherapy was made through the discussion with patients, medical oncologist, and radiation oncologist. The severity of complications was graded using the Clavien-Dindo (CD) classification [
11]. SE level was measured at the following periods; preoperatively, 7 days postoperatively, and 6 months or 12 months postoperatively.
Definition of subgroups
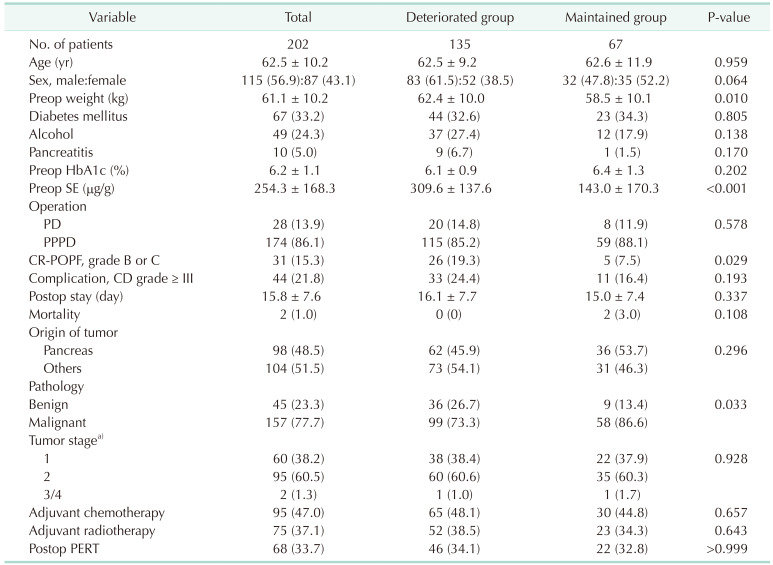
Patients with SE levels less than 100 µg/g were considered to have severe EPI. The deteriorated (exocrine function) group comprised patients whose SE level decreased from 100 µg/g or more in the preoperative period to less than 100 µg/g at 6 months or 12 months postoperatively. The remaining patients were classified in the maintained group. We compared clinical and nutritional parameters between the 2 groups.
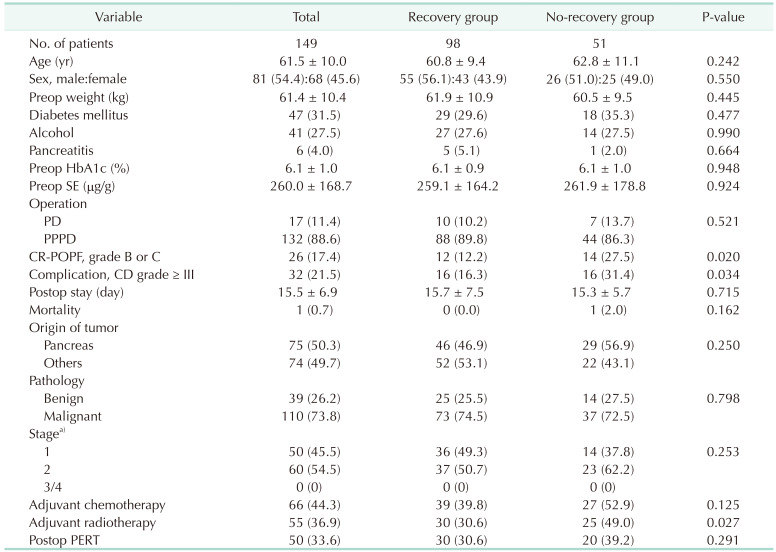
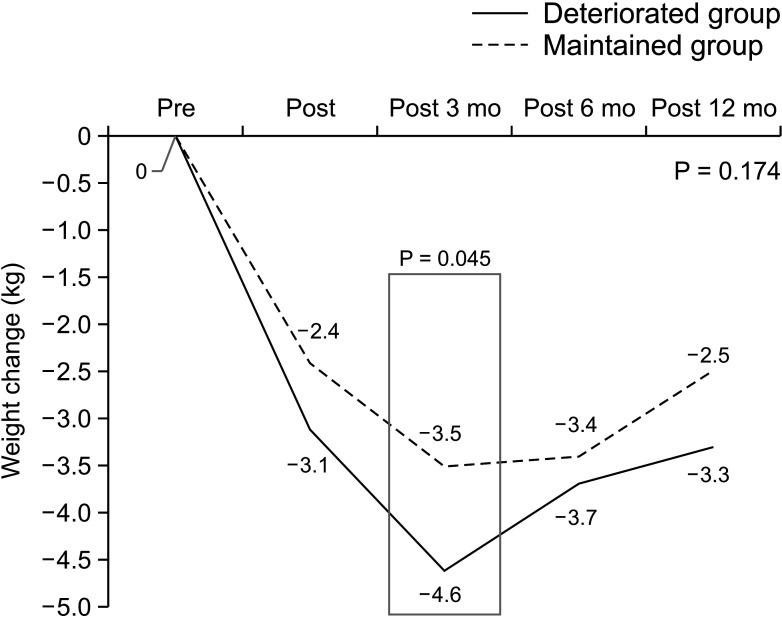
For serial weight comparison, we divided the patients into 2 groups based on their weight recovery. Measurements at 3 and 12 months postoperatively were performed in enrolled patients. When comparing the 2 figures, if the weight 12 months postoperatively was greater than that at 3 months postoperatively, patients were classified in the recovery group. The rest of the patients were classified into the no-recovery group.
Statistical analysis
The chi-square test or Fisher exact test was used to compare categorical variables. The normality of distribution was assessed for all continuous variables, following which they were compared between the groups using the Student t-test or Mann-Whitney U-test. Logistic regression analysis was performed to identify risk factors for severe EPI. A repeated analysis of variance was used to compare the weight change and nutritional parameters of the deteriorated and maintained groups. Statistical significance was set at P < 0.05. IBM SPSS Statistics for Windows, ver. 22.0 (IBM Corp. Armonk, NY, USA) was used for statistical analyses.
Go to :

DISCUSSION
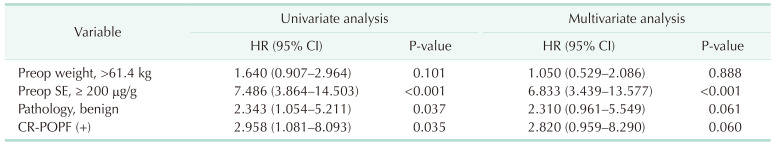
The intensive evaluation and management of postoperative EPI are required as both preoperatively healthy patients in normal condition and those already experiencing EPI status suffer from rapid deterioration of exocrine function after surgery. The conventional method for evaluating EPI is measuring the SE level. Patients with preoperative SE levels above 200 µg/g, benign disease, and CR-POPF suffer from the deterioration of exocrine insufficiency after surgery, and patients who underwent adjuvant radiotherapy had difficulty recovering their weight after surgery.
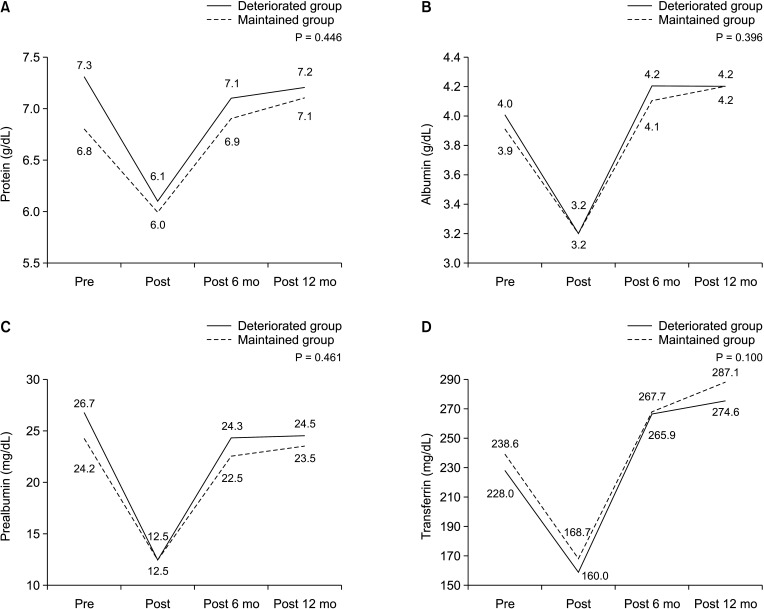
The aim of this study was to analyze the risk factors for deteriorated exocrine insufficiency after PD by measuring the SE levels. A preoperative SE level of > 200 µg/g, benign pathology, and the presence of CR-POPF were identified as risk factors for EPI. Between-group differences in postoperative weight changes were not significant; these were also not significant for the nutritional parameters for up to 1 year postoperatively. This implies that the SE level does not reflect the nutritional status, which includes the weight and nutritional parameters. In addition, the risk factor for difficulty in weight recovery was the history of postoperative radiotherapy, which was different from the risk factors for deteriorated exocrine insufficiency. This suggests that evaluating the patients’ nutritional status by merely measuring the SE level is not sufficient. A comprehensive approach for the evaluation of exocrine function is required that includes not only the SE level but also other nutritional parameters.
The method of measuring SE-1 used in this study is the most commonly used indirect method. It employs an ELISA technique using monoclonal or polyclonal antibodies. Elastase 1 is a proteolytic enzyme produced in pancreatic acinar cells that bind to bile salts and passes through the gut. It lacks relevant degradation; thus, it is highly stable in the intestinal tract and is eventually measurable in fecal samples [
121314]. The concentration of this enzyme is 5 times stronger in the feces than in the pancreatic juice. It is an indicator for pancreatic output, is stable at room temperature for up to 1 week, and can be stored at 4°C for 1 month [
15]. In summary, currently, the most useful method to evaluate pancreatic exocrine function in clinical settings is the serial evaluation of the SE level.
Park et al. [
16] analyzed patients who underwent PD or distal pancreatectomy and reported that the SE level decreased after surgery and remained low throughout the 12 postoperative months. The mean SE level was lowest at 3 months postoperatively and did not recover after 3, 6, and 12 months postoperatively. However, the EPI symptoms, steatorrhea, and diarrhea scores gradually decreased at 3, 6, and 12 months postoperatively. Additionally, Kim et al. [
10] reported that SE tended to decrease after surgery and remained unrecovered 12 months after surgery. The participants’ body mass index and nutritional status decreased immediately after surgery and was lower at 12 months postoperatively compared with the preoperative level. When compared based on the change in SE level as in this study, as well as the absolute value of SE level in the aforementioned studies, the evaluation of EPI exclusively by measuring the SE level is insufficient and other parameters should be considered.
Several factors can cause postoperative exocrine insufficiency. It is caused by the extent of pancreatic resection and the presence of fibrosis or atrophy, which suggests preexisting obstructive pancreatitis [
1718]. Accelerated gastric emptying and intestinal transit may occur after pancreatic resection, which causes asynchrony and inadequate mixing of the enzyme, resulting in secondary exocrine insufficiency [
1920]. Changes in gastric and duodenal acidity also induce inactivation of pancreatic enzyme [
221]. Cholecystokinin is secreted from the duodenal mucosa and plays a role in promoting pancreatic secretion. After PD, the abnormal secretion of postprandial cholecystokinin reduces exocrine pancreatic secretion. Obstruction of an anastomosis may occur because of tumor recurrence and fibrosis [
22]. Pathologically benign masses are also associated with severe EPI after PD [
23]. In case of malignant disease, pancreatic function may have changed gradually due to obstruction of pancreatic duct and fibrotic change of pancreas parenchymal tissues resulted by repetitive inflammation. However, in case of benign disease, surgery would have caused a sudden decline of normal pancreatic function. Thus, exocrine function gradually declines over a relatively long period of time in malignant disease than in benign disease. It has been reported that the size, localization, ductal involvement, and surgical intervention for the masses may affect exocrine function in cases of neuroendocrine tumors and benign pancreatic cysts [
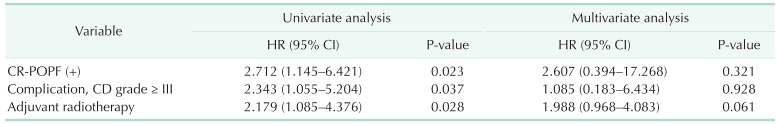
23]. Further, the presence of CR-POPF has been identified as a risk factor for deteriorated exocrine insufficiency. Patients in the no weight-recovery group had a higher proportion of CR-POPF, a higher CD complication grade, and incidence of history of adjuvant radiotherapy than those in the recovery group. Therefore, doctors should continue to pay attention to the nutritional aspects of patients even after the recovery of complications.
Patients with EPI or exocrine insufficiency-related symptoms including steatorrhea, diarrhea, or weight loss are generally administered PERT [
17]. According to a randomized, multicenter trial, PD patients with an SE level <200 µg/g preoperatively or postoperatively were randomly assigned to the PERT group or placebo group. The PERT group achieved a weight gain of 1.09 kg and placebo group suffered weight loss of 2.28 kg at 3 months, resulting in significant difference (P < 0.001). Poor compliance with PERT was a significant risk factor for weight loss (P < 0.001). In addition, PERT caused an increase in prealbumin and transferrin levels at 3 months postoperatively [
24]. However, in our study, PERT did not have a significant impact on weight recovery after surgery. There are some possible reasons for this. There was no definite indication for PERT was in the clinical practice, and the patients’ compliance with PERT was not measured in the outpatient clinic. In addition, the dosage of pancreatic enzyme supplement used in this study was 25,000 IU, which is lower than the dosage generally used in clinic settings, which is 40,000 IU. Moreover, patients’ dietary habits were not included in this study. People with meat-based eating habits require a higher dose of PERT than those with vegetarian eating habits. It is possible that the PERT dosage in our study did not correspond with the eating habits; therefore, PERT was not sufficient to affect weight recovery after surgery. Our study can be converted to a prospective long-term study by including indications and compliance with PERT, diet habits, and dosage of PERT as factors. Thus, it would be possible to conduct a prospective long-term study.
A limitation of this study is that it was a retrospective study, and even though it is based on prospectively collected data, the study design may eventually affect the results. In addition, EPI was evaluated only by measuring the SE. The aforementioned limitations of SE render the criteria for grouping the deteriorated and maintained groups unreasonable. As we focused on the patients who suffered rapid change in pancreatic exocrine function, we had no choice but to include patients whose SE level was less than 100 µg/g in the preoperative period. If we exclude patients whose preoperative SE level is less than 100 µg/g, 47 patients are excluded from the maintained group. When we analyze 135 patients of the deteriorated group and 20 patients of the new maintained group as a subgroup analysis, there is a high discrepancy of number of patients in the 2 groups. In the same way, the presence of adjuvant radiotherapy (P = 0.022) is the risk factor of deteriorated exocrine function in subgroup analysis. Also, serial postoperative changes and serial nutritional parameters are not significantly different between the 2 groups. In addition, among patients who underwent PD for periampullary disease at SNUH from October 2007 through February 2013, a number of patients who were not checked preoperative or postoperative SE levels were excluded. The final enrollment was 202 patients; 342 patients who were in exclusion criteria were missing data. For this reason, factors that were previously shown to be statistically meaningful were not statistically significant.
However, this study has several strengths. It specifically dealt with EPI after PD, which has been rarely reported on until now. We also conducted our study on weight recovery after PD through a subgroup analysis and identified the risk factors influencing weight recovery. Finally, the serial assessment of weight, SE, and nutritional parameters of patients for 1 year made it possible to achieve the results.
In conclusion, patients with preoperative SE levels above 200 µg/g, benign disease, and CR-POPF suffer from deterioration of exocrine insufficiency after surgery. Patients who underwent adjuvant radiotherapy had difficulty weight recovery after surgery. The risk factors for the deterioration of exocrine insufficiency and weight change were different. This discrepancy suggests that measurement of only the SE level is not sufficient to evaluate the actual nutritional status of patients. In other words, a comprehensive approach including serial SE level, weight change, and nutritional parameters is mandatory for the evaluation of EPI. Further studies can enable the optimal management of EPI after PD by including data on the administration of PERT and dietary habits of the patients.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download