Abstract
Purpose
The standard of care for early rectal cancer is radical surgery; however, it carries high postoperative morbidity. This study aimed to assess the short-term and oncological outcomes of local excision and adjuvant radiotherapy in patients with high-risk pathological stage (p) T1 rectal cancer.
Methods
Fifty-five patients underwent local excision with adjuvant radiotherapy or radical resection for high-risk T1 rectal cancer. Patients with adenocarcinoma within 10 cm from the anal verge; pT1 with high-risk features (grade 3–4); a tumor size of ≥3 cm; a positive margin; a lymphovascular or perineural invasion; or a submucosal invasion depth of ≥SM2 were included.
Results
The rates of postoperative complications and stoma formation were higher in the radical surgery group (P = 0.021 and P = 0.003, respectively). No significant differences were observed in the overall survival and disease-free survival (DFS) between the 2 groups (P = 0.301 and P = 0.076, respectively). Vascular invasion was a significantly poor prognostic factor for DFS (P = 0.033). The presence of 3 or more high-risk features was associated with a poor DFS (P = 0.002).
The standard of care for early rectal cancer is radical surgery, namely, total mesorectal excision (TME), which carries a high risk of postoperative morbidities and influences the patients’ quality of life [1]. Low anterior resection syndrome and stoma formation can negatively affect bowel function [23], along with various aspects of sexual dysfunction, urological difficulties, and psychological disturbances [1].
Local excision for early rectal cancer was restricted to patients with contraindications to radical surgery or to accept procedure-related recurrence and death [4]. However, with the advancements in early rectal cancer assessment modalities, the improvements in the techniques used to perform local excision and adjuvant therapy for selected patients, and the decreased postoperative morbidity and incidence of stoma formation, local excision has become an alternative to radical surgery [56]. However, with the advances in early rectal cancer assessment modalities, some centers perform transanal endoscopic microsurgery (TEM) for full-thickness assessment due to the uncertainty of the preoperative diagnosis and staging [7].
Several studies have reported a relatively high recurrence rate after transanal local excision (TLE) for early rectal cancer [8910]. The primary concern associated with disease recurrence is the presence of high-risk histopathological features, which increases the risk of lymph node involvement and recurrence. This concern has been studied extensively over the past few decades. Researchers have reported that cancer type, lymphovascular invasion (LVI), depth, and invasion width carry a high recurrence risk [1112]. However, adjuvant chemoradiotherapy significantly decreases the incidence of local recurrence [13]; to achieve this low recurrence rate, multidisciplinary treatment is needed for selected patients and an intensive surveillance program must be implemented [1415]. Interestingly, Borstlap et al. [16] reported in their meta-analysis that the local recurrence rates in T1 rectal cancer between patients with TLE with adjuvant therapy and those who underwent radical surgery were comparable.
As only a few studies in the literature have addressed the usefulness of radiotherapy after local excision for early rectal cancer, we aimed to assess the postoperative complications and morbidity rates of radiation therapy following local excision in pathological stage (p) T1 rectal adenocarcinoma patients with unfavorable histological features, and compare the oncological outcomes with those of the radical resection group.
A retrospective analysis of the medical records of 78 early rectal cancer patients who underwent local excision followed by adjuvant radiotherapy or radical surgery for T1 rectal cancer with high-risk histological features was performed from April 2010 to July 2020 at Yeouido St. Mary’s Hospital, Seoul St. Mary’s Hospital, and St. Vincent Hospitals. This study was approved by the Institutional Review Board of the College of Medicine, The Catholic University of Korea (No. KC21RASI0391). It was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
Patients with a histologically confirmed adenocarcinoma within 10 cm from the anal verge (AV); pT1 high-risk features, including a poorly or undifferentiated adenocarcinoma; a tumor size of ≥3 cm; a positive resection margin; a lymphatic, vascular, or perineural invasion; or a submucosal invasion of ≥SM2 were included in our study. Patients who had low-risk T1 rectal cancer, carcinoma in situ (Tis), another primary cancer, upper rectal cancer, or refusing adjuvant radiotherapy after TLE were excluded from our analysis (Fig. 1).
Endoscopic biopsy, polypectomy, endoscopic mucosal resection, or endoscopic submucosal dissection (ESD) was performed prior to attempting local excision. We reviewed the histopathological results of biopsy specimens to obtain an accurate diagnosis and to assess for unfavorable features. Clinical and radiological assessments were performed, including digital rectal examination and proctoscopy, to identify the lesion size, location, and accessibility for transanal excision plus abdominopelvic CT and pelvic MRI for staging. Therefore, local excision is considered for both diagnostic and therapeutic purposes. However, some patients underwent upfront radical resection due to patient’s preference or surgeon’s experience (Fig. 2).
A multidisciplinary team reviewed the final histopathological results of local excision to determine whether the patient was suitable for adjuvant radiotherapy or radical surgery according to the histopathological features and comorbidity, considering the patient’s preference and surgeon’s experience.
After 3 months, the patients who underwent local excision and adjuvant radiotherapy underwent follow-up sigmoidoscopy and abdominopelvic CT. When there are no signs of recurrence, the patients underwent sigmoidoscopy and abdominopelvic CT every 3 months for the first 3 years, every 6 months for the next 2 years, and then annually. Clinical and endoscopic evaluation, plus carcinoembryonic antigen testing, abdominopelvic CT, or pelvic MRI were carried out at each visit.
Data on patient’s demographic characteristics (sex, age, and body mass index [BMI]); tumor size; distance from the AV; margin and submucosal invasion; histological types; lymphatic, vascular, and perineural invasion; duration of follow-up; recurrence; postoperative complications; and stoma status were obtained.
Short-term outcomes were defined as morbidity that occurred during the first 30 days postoperatively. Meanwhile, long-term outcomes referred to the oncological outcomes, including recurrence and metastasis. Overall survival (OS) was defined as the period from the date of surgery to the time of death. Patients were censored at the date that they were last known to be alive (last clinic or investigation attendance). Disease-free survival (DFS) was defined as the period from the date of surgery to the time of disease recurrence (local or distant).
Categorical variables were analyzed using the chi-square test. Continuous variables were expressed as mean ± standard deviation and were compared using the Student t-test. Patients’ characteristics and follow-up results were compared between the local and radical groups; the DFS was analyzed using the Kaplan-Meier curve. Statistical significance was set at P < 0.05. Statistical analyses were performed using IBM SPSS Statistics ver. 24.0 (IBM Corp., Armonk, NY, USA).
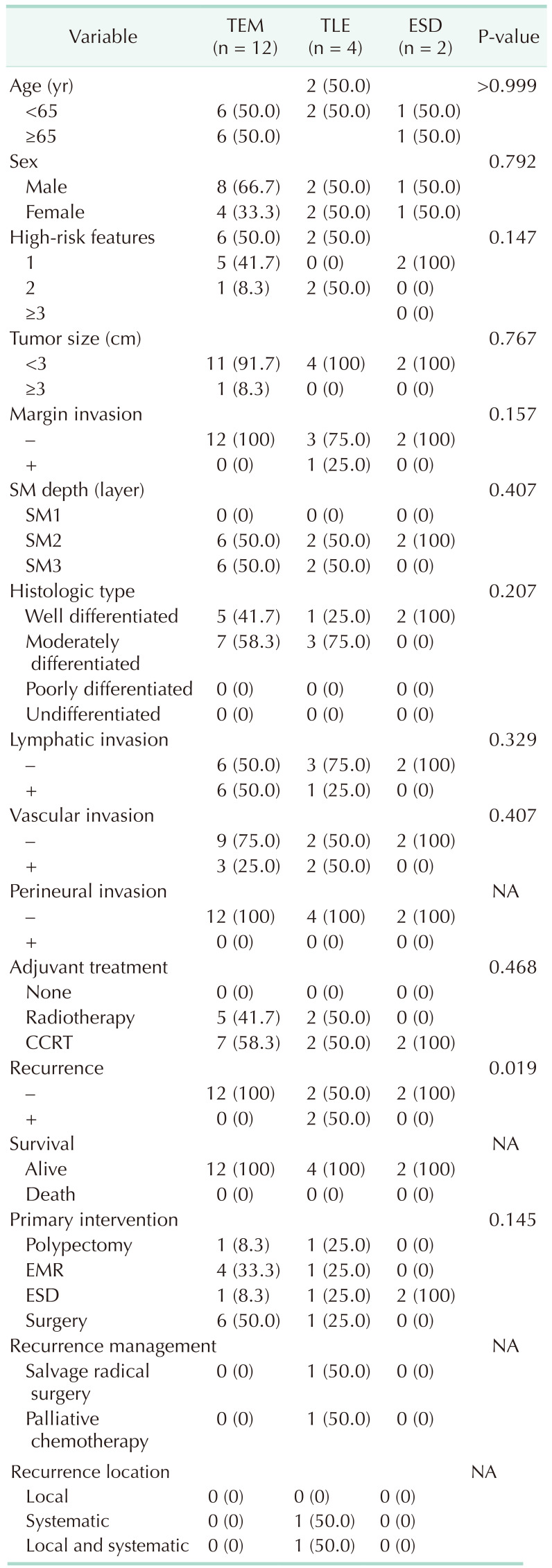
Fifty-five patients were enrolled in this study and were divided into the radical resection group (37 patients) and local excision group (18 patients). Their mean age was 61.98 ± 9.97 years; of the total participants, 65.5% were male and 34.5% were female. No difference was found in the baseline characteristics (age, sex, and BMI). The mean tumor distances from the AV were 7.24 ± 2.5 cm and 4.83 ± 2.5 cm in the radical group and local excision group, respectively, which showed a significant difference (P < 0.002). In the radical group, 1 patient underwent an abdominoperineal resection (APR), 4 underwent an intersphincteric resection, and 32 underwent a low anterior resection (Table 1). In the local excision group, 12 patients underwent a TEM, 4 underwent a TLE, and 2 underwent an ESD. No difference was found in the baseline characteristics among the different approaches of local excision (Table 2).
The high-risk features were measured; no significant difference was found between the radical and local excision groups. Furthermore, no difference was observed in the high-risk features in contrast to the different local excision approaches. One patient with a positive resection margin was identified (5.6%) in the local excision group after TLE; however, the difference was not significant compared with the radical resection group. No difference was found in the histological types. The LVI in the local excision group was insignificant compared with that in the radical resection group, which had seven patients with positive lymphatic invasion and five with vascular invasion. With regard to the submucosal depth of invasion, a significant difference was observed between the 2 groups. In the radical resection group, 2 patients (5.4%) had SM2 invasion, while 33 (89.2%) had SM3 invasion. In the local excision group, 10 patients (55.6%) had SM2 and 8 (44.4%) had SM3 invasion (P < 0.001) (Table 1).
Within the 30-day postoperative period, 10 patients (27.0%) in the radical surgery group developed some complications (ileostomy prolapse, 1; anastomotic leakage, 1; anastomotic stricture, 1; left ureteric injury, 1; ileus, 2; wound infection, 1; neuropathic bladder, 1; erectile dysfunction, 1; and fecal incontinence due to high stool frequency, 1). However, no complications were reported in the local excision group, indicating a significant difference between the 2 groups (P = 0.021). Moreover, the rates of stoma formation were significantly higher in the radical surgery group than in the local excision group (P = 0.003). A total of 17 patients (45.9%) had a temporary stoma in the radical surgery group, while none in the local excision group. By contrast, one patient in each group had a permanent stoma. Meanwhile, none of the patients in either group died 30 days after the surgery.
The overall median follow-up was 52.78 ± 34.46 months (60.68 ± 33.40 months in the radical group and 36.56 ± 31.53 months in the local excisional group, P = 0.013). Clinical recurrence was not significantly different between radical excision and local excision with adjuvant therapy (P = 0.103). However, 2 patients who underwent local excision experienced disease recurrence. Specifically, recurrence occurred in the TLE subgroup but not after undergoing TEM. One patient had distant metastasis after 41 months of follow-up, while 1 patient had local recurrence with distant metastasis after 14 months of follow-up. The patient who had local recurrence underwent salvage radical resection with a permanent stoma, and the other patient with distant metastasis received palliative chemotherapy. These 2 patients had 3 high-risk features based on their histopathological reports. One patient had submucosal (SM3) depth invasion, vascular invasion, and mucinous component. The second patient had positive excisional margins, lymphatic invasion, and vascular invasion (Table 2).
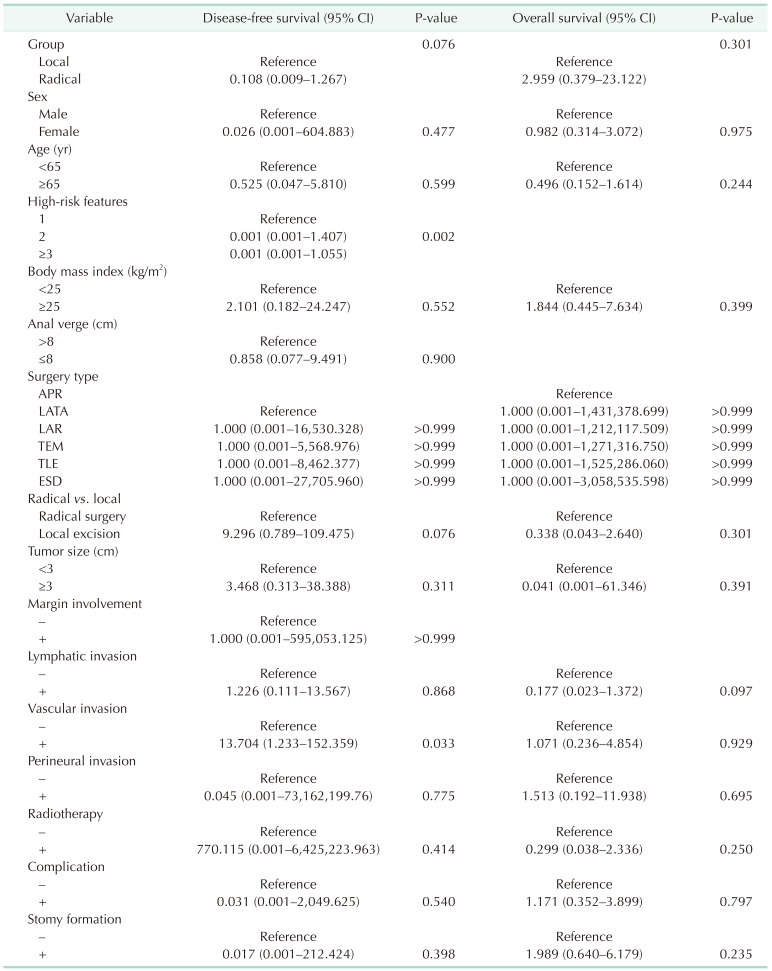
The OS rate was similar in both groups (P = 0.301). With regard to the DFS, the analysis showed that the radical group had a superior tendency compared with the local excision group, but the difference was not significant (P = 0.076) (Table 3). Vascular invasion was a significantly poor prognostic factor for DFS (P = 0.033). Presence of 3 or more high-risk features was associated with poor DFS (P = 0.002) (Fig. 3).
The management of rectal cancer has improved over the past few decades, and TME without neoadjuvant or adjuvant therapy remains the cornerstone of therapy for early rectal cancer, associated with a decreased incidence of local recurrence and subsequent improvements in patient’s survival. Thus, our data showed no recurrence of unfavorable T1 rectal cancer in the radical resection group. Mellgren et al. [17] reported that within the 5-year follow-up, 18% of the patients in the local excision group and none in the radical resection group developed local recurrence. Nevertheless, rectal preservation benefits, low morbidity, less operative time, and the reduced length of hospital stay have made a clinical shift toward less invasive approaches [18].
As no conclusive criteria have been established yet, the selection of patients who will undergo local excision or radical resection remains a challenge during the preoperative period. After conducting an endoscopic biopsy, we aimed to implement local excision in any patient with clinical and radiological T1 rectal cancer to determine the underlying pathology. We revised the criteria used for selecting patients for undergoing local excision and adjuvant radiotherapy. However, clinicians should carefully select the suitable patients and educate them regarding the possible interventions that would be implemented and the complications that might occur, as the oncological outcomes for salvage radical resection after local excision are poorer than those of the initial radical resection [8].
The different techniques for local excision applied in this study depend on the surgeon’s experience and preference. Approximately 12 of the patients from the local excision group (66.7%) underwent TEM performed by the same surgeon. Indeed, TEM is more effective in obtaining negative excisional margins and better DFS than TLE. A meta-analysis [19] of 11 studies included 1,191 patients, who were divided into the TEM group (514 patients), radical resection group (291 patients), and the TLE group (386 patients). Results of the meta-analysis showed that radical resection was more superior in obtaining a negative margin; moreover, TLE was reported to be inferior to TEM. The TLE subgroup’s recurrence rate in the present study was consistent with that in the previous studies, which reported a higher recurrence rate after conducting a TLE [8].
Despite the recent advances in local excision techniques, unfavorable histological features are associated with the risk of lymph node metastasis and local recurrence. Therefore, the assessment of regional lymph nodes after a local excision remains challenging. Nascimbeni et al. [11] reported that the depth of invasion and LVI were associated with a significant risk of lymph node metastasis. On the contrary, Kikuchi et al.’s study [20] showed that lymph node metastasis was related to the depth of submucosal invasion, unlike LVI. In comparison, Min et al. [21] reported LVI and SM2 invasion as risk factors for local recurrence. However, several studies have concluded that the rate of lymph node metastasis and local recurrence is higher in patients with early rectal cancer than in those with colonic cancer [202223]. In our study, we found that vascular invasion had a clinical significance (P = 0.033). Interestingly, the 2 patients who experienced a recurrence were found to have 3 high-risk features. The first patient had a lower third submucosal invasion (SM3), vascular invasion, and mucinous component, while the second patient had positive resection margins, lymphatic invasion, and vascular invasion.
In this study, patients who underwent local excision received adjuvant radiotherapy either alone or in combination with concurrent chemotherapy-accepting a higher recurrence rate after local excision against the morbidity of radical resection. A previous meta-analysis [16] showed that the recurrence rate after a local excision with adjuvant radiotherapy for high-risk early rectal cancer is 2-fold higher than that after radical resection. Therefore, an intensive surveillance program for these patients is crucial. Nevertheless, recent studies have shown promising results regarding the effectiveness of radiotherapy when combined with TEM [24]. Duek et al. [25] found no recurrence at a median follow-up of 3 years after TEM with adjuvant radiotherapy in 12 patients with early rectal cancer. Balyasnikova et al. [5] found no recurrence at a median follow-up of 4 years in 18 patients with high-risk rectal cancer after undergoing a local excision with adjuvant radiotherapy. Our data also showed no recurrence at a median follow-up of 3 years in the TEM subgroup treated with adjuvant radiotherapy.
Recently, there has been an increasing interest in the nonoperative “watch and wait” approach. However, before implementing this approach, the possible oncological outcomes must be determined. These patients have the chance to achieve excellent results with very low recurrence rates with neoadjuvant therapy and radical resection. The primary challenge and pitfall of this strategy are that it may leave a viable residual tumor in the rectum of a patient who achieved a complete clinical response but not a complete pathological response. Habr-Gama et al. [26] reported that the risk of locoregional treatment failure of the watch and wait approach was approximately 30%. Other studies have shown a 50%–60% risk of locoregional treatment failure [2728]. A meta-analysis concluded that a universal criterion for response assessment needs to be established to evaluate the safety of this strategy [29]. Considering that this strategy is used in advanced cancer, local excision followed by adjuvant radiotherapy may be an alternative treatment option for patients with early cancer with high-risk features.
The short-term benefits of local excision must be counter-balanced with its oncological outcomes. Uncertainty persists concerning the oncological appropriateness of local excision for rectal cancer. In our study, no complications were reported following the different local excision techniques or temporary stoma maturation (P = 0.021 and P = 0.003, respectively). However, a permanent stoma was made in the local excision group due to local recurrence, which was managed with APR. By contrast, recurrence occurred in 2 patients from the TLE subgroup who had more than 2 high-risk features in their final histopathological evaluation. The first patient experienced local recurrence detected during surveillance and was managed with radical resection. Later, the patient developed distant metastasis to the lungs, ribs, and vertebrae and underwent palliative chemotherapy. The second patient had lung metastasis on follow-up and received systemic chemotherapy. Thus, the group who had radical resection showed a superior tendency to develop recurrence compared with the group who had local excision with radiotherapy, but the difference was not significant (P = 0.076). This result is consistent with that of previously published studies.
The present study has some limitations. First, our review was retrospective in nature. Second, the surgical intervention depends on the surgeon’s experience and patient preference; therefore, the potential of selection bias exists. Third, it has a limited sample size. Lastly, we defined SM1 as tumor invasion in the submucosa of less than 1,000 µm, SM2 as equal to 1,000 µm or more but less than 2,000 µm, and SM3 as equal to 2,000 µm or more. Thus, the pathological results of the 3 hospitals were not standardized. However, the results are promising for patients with less than 3 high-risk T1 rectal cancer features who prefer local excision and adjuvant radiotherapy instead of radical resection to avoid procedure morbidity and improve the quality of life; in order to achieve an acceptable low risk of recurrence, a meticulous surveillance must be mandated.
Our study concludes that local excision with adjuvant radiotherapy is a better treatment option to avoid complications and stoma formation. Furthermore, local excision with adjuvant radiotherapy is an alternative option for patients with T1 rectal cancer with fewer than 3 high-risk features.
References
1. Borstlap WA, Tanis PJ, Koedam TW, Marijnen CA, Cunningham C, Dekker E, et al. A multi-centred randomised trial of radical surgery versus adjuvant chemoradiotherapy after local excision for early rectal cancer. BMC Cancer. 2016; 16:513. PMID: 27439975.

2. Croese AD, Lonie JM, Trollope AF, Vangaveti VN, Ho YH. A meta-analysis of the prevalence of Low Anterior Resection Syndrome and systematic review of risk factors. Int J Surg. 2018; 56:234–241. PMID: 29936195.

3. Ha RK, Park SC, Park B, Park SS, Sohn DK, Chang HJ, et al. Comparison of patient-reported quality of life and functional outcomes following laparoscopic and transanal total mesorectal excision of rectal cancer. Ann Surg Treat Res. 2021; 101:1–12. PMID: 34235111.

4. Nash GM, Weiser MR, Guillem JG, Temple LK, Shia J, Gonen M, et al. Long-term survival after transanal excision of T1 rectal cancer. Dis Colon Rectum. 2009; 52:577–582. PMID: 19404055.

5. Ba lyasnikova S, Read J, Tait D, Wotherspoon A, Swift I, Cunningham D, et al. The results of local excision with or without postoperative adjuvant chemoradiotherapy for early rectal cancer among patients choosing to avoid radical surgery. Colorectal Dis. 2017; 19:139–147. PMID: 27474876.

6. Hwang Y, Yoon YS, Bong JW, Choi HY, Song IH, Lee JL, et al. Long-term transanal excision outcomes in patients with T1 rectal cancer: comparative analysis of radical resection. Ann Coloproctol. 2019; 35:194–201. PMID: 31487767.

7. Morino M, Allaix ME. Transanal endoscopic microsurgery: what indications in 2013? Gastroenterol Rep (Oxf). 2013; 1:75–84. PMID: 24759812.

8. You YN, Baxter NN, Stewart A, Nelson H. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Ann Surg. 2007; 245:726–733. PMID: 17457165.
9. Patel SA, Chen YH, Hornick JL, Catalano P, Nowak JA, Zukerberg LR, et al. Early-stage rectal cancer: clinical and pathologic prognostic markers of time to local recurrence and overall survival after resection. Dis Colon Rectum. 2014; 57:449–459. PMID: 24608301.
10. Stornes T, Wibe A, Nesbakken A, Myklebust TÅ, Endreseth BH. National early rectal cancer treatment revisited. Dis Colon Rectum. 2016; 59:623–629. PMID: 27270514.

11. Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002; 45:200–206. PMID: 11852333.

12. Toh EW, Brown P, Morris E, Botterill I, Quirke P. Area of submucosal invasion and width of invasion predicts lymph node metastasis in pT1 colorectal cancers. Dis Colon Rectum. 2015; 58:393–400. PMID: 25751795.

13. Cutting JE, Hallam SE, Thomas MG, Messenger DE. A systematic review of local excision followed by adjuvant therapy in early rectal cancer: are pT1 tumours the limit? Colorectal Dis. 2018; 20:854–863. PMID: 29992729.

14. Suzuki T, Sadahiro S, Tanaka A, Okada K, Saito G, Miyakita H, et al. Outcomes of local excision plus chemoradiotherapy in patients with T1 rectal cancer. Oncology. 2018; 95:246–250. PMID: 29909419.

15. Jones HJ, Goodbrand S, Hompes R, Mortensen N, Cunningham C. Radiotherapy after local excision of rectal cancer may offer reduced local recurrence rates. Colorectal Dis. 2019; 21:451–459. PMID: 30585677.

16. Borstlap WA, Coeymans TJ, Tanis PJ, Marijnen CA, Cunningham C, Bemelman WA, et al. Meta-analysis of oncological outcomes after local excision of pT1-2 rectal cancer requiring adjuvant (chemo)radiotherapy or completion surgery. Br J Surg. 2016; 103:1105–1116. PMID: 27302385.

17. Mellgren A, Sirivongs P, Rothenberger DA, Madoff RD, García-Aguilar J. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000; 43:1064–1074. PMID: 10950004.

18. Palma P, Horisberger K, Joos A, Rothenhoefer S, Willeke F, Post S. Local excision of early rectal cancer: is transanal endoscopic microsurgery an alternative to radical surgery? Rev Esp Enferm Dig. 2009; 101:172–178. PMID: 19388797.

19. Sgourakis G, Lanitis S, Gockel I, Kontovounisios C, Karaliotas C, Tsiftsi K, et al. Transanal endoscopic microsurgery for T1 and T2 rectal cancers: a meta-analysis and meta-regression analysis of outcomes. Am Surg. 2011; 77:761–772. PMID: 21679648.

20. Kikuchi R, Takano M, Takagi K, Fujimoto N, Nozaki R, Fujiyoshi T, et al. Management of early invasive colorectal cancer: risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995; 38:1286–1295. PMID: 7497841.
21. Min BS, Kim NK, Ko YT, Lee KY, Baek SH, Cho CH, et al. Long-term oncologic results of patients with distal rectal cancer treated by local excision with or without adjuvant treatment. Int J Colorectal Dis. 2007; 22:1325–1330. PMID: 17571241.

22. Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology. 1985; 89:328–336. PMID: 4007423.

23. Sticca RP, Rodriguez-Bigas M, Penetrante RB, Petrelli NJ. Curative resection for stage I rectal cancer: natural history, prognostic factors, and recurrence patterns. Cancer Invest. 1996; 14:491–497. PMID: 8816864.

24. Hakiman H, Pendola M, Fleshman JW. Replacing transanal excision with transanal endoscopic microsurgery and/or transanal minimally invasive surgery for early rectal cancer. Clin Colon Rectal Surg. 2015; 28:38–42. PMID: 25733972.

25. Duek SD, Issa N, Hershko DD, Krausz MM. Outcome of transanal endoscopic microsurgery and adjuvant radiotherapy in patients with T2 rectal cancer. Dis Colon Rectum. 2008; 51:379–384. PMID: 18236108.

26. Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silva e Sousa AH Jr, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004; 240:711–718. PMID: 15383798.
27. Nakagawa WT, Rossi BM, de O Ferreira F, Ferrigno R, David Filho WJ, Nishimoto IN, et al. Chemoradiation instead of surgery to treat mid and low rectal tumors: is it safe? Ann Surg Oncol. 2002; 9:568–573. PMID: 12095973.

28. Dalton RS, Velineni R, Osborne ME, Thomas R, Harries S, Gee AS, et al. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis. 2012; 14:567–571. PMID: 21831177.

29. Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017; 2:501–513. PMID: 28479372.

Fig. 1
A flowchart of the research. A total of 78 patients were enrolled in the study, 23 patients were excluded; 37 patients (67.3%) underwent radical resection, and 18 patients (32.7%) had local excision with adjuvant radiotherapy. pT1, pathological stage T1.

Fig. 2
After reviewing colonoscopic biopsy, patients underwent local excision or upfront radical resection depending on patients’ preference and surgeon experience. EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; TEM, transanal endoscopic microsurgery; TLE, transanal local excision; MDT, multidisciplinary team.

Fig. 3
Disease-free survival (DFS) for (A) local excision and radical resection groups, (B) vascular invasion as a risk factor, and (C) numbers of high-risk features factors.

Table 1
Patients’ characteristics
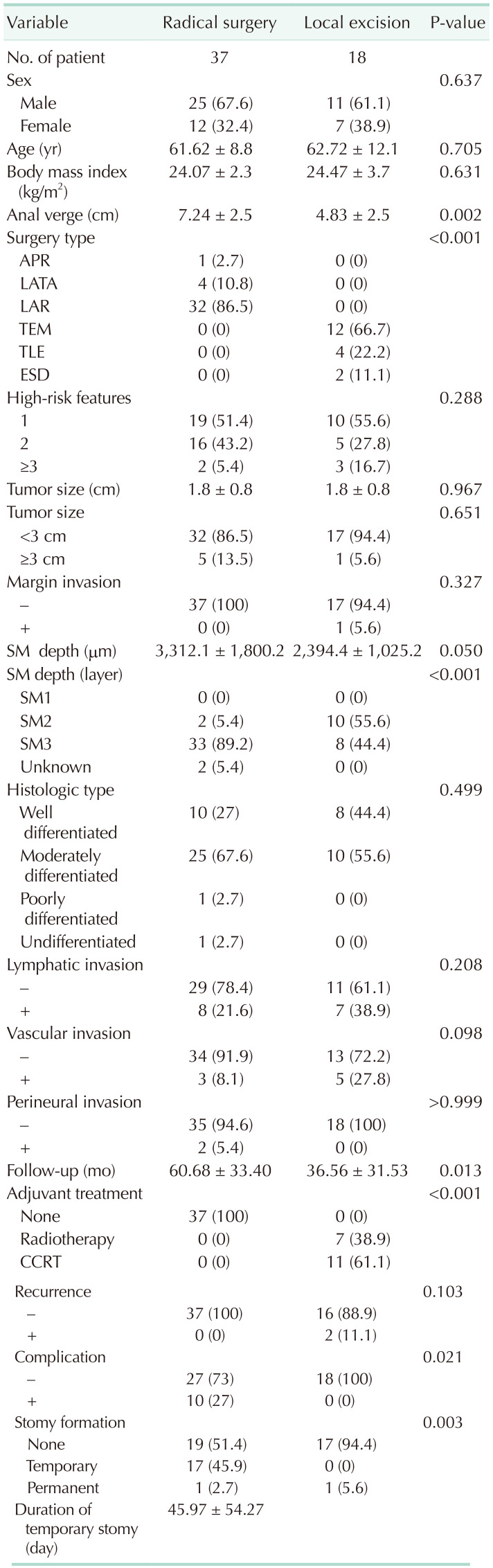
Values are presented as number only, number (%), or mean ± standard deviation.
APR, abdominoperineal resection; LATA, laparoscopic abdominal transanal proctosigmoidectomy; LAR, low anterior resection; TEM, transanal endoscopic microsurgery; TLE, transanal local excision; ESD, endoscopic submucosal dissection; SM, submucosa; CCRT, concurrent chemoradiation therapy.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download