Abstract
Angiodysplasia (AD) is increasingly being recognized as a major cause of gastrointestinal bleeding. Morphologically flat lesions are common types of AD, whereas the polypoid types are rare. We report a case of multiple polypoid AD in the small bowel causing severe anemia and requiring surgical treatment. A 60-year-old male patient visited our hospital with dyspnea and hematochezia. He had a history of myocardial infarction and was taking both aspirin and clopidogrel. Capsule endoscopy, enteroscopy, computed tomography, and angiography revealed multifocal vascular lesions with a polypoid shape in the jejunum. Surgical resection was performed because endoscopic treatment was considered impossible with the number and the location of lesions. The risk of recurrent bleeding related to the use of antiplatelet agents also contributed to the decision to perform surgery. AD was histologically diagnosed from the surgical specimen. He resumed taking both aspirin and clopidogrel after surgery. He fully recovered and has been doing well during the several months of follow-up.
Angiodysplasia (AD) is the most common vascular lesion in the gastrointestinal (GI) tract. Although the cecum and the ascending colon are the most common sites of AD, in 15% of cases, AD is assumed to occur in the small bowel [1]. It has been demonstrated that bleeding in the colon is mild and spontaneously resolves in up to 90% of patients. However, lesions in the small bowel can cause occult yet severe GI bleeding that can be fatal [2].
Vascular lesions in the GI tract are one of the most important causes of GI bleeding. They are generally categorized as either neoplastic lesions or nonneoplastic malformations. Owing to their overlapping characteristics, the classification of vascular lesions requires various modalities, including endoscopic, radiologic, and histologic evaluations. Neoplastic lesions include hemangioma whose endoscopic findings include polypoid, compressible, and bluish features [3]. AD and arteriovenous malformation (AVM) are considered nonneoplastic malformations.
We here report a case of multiple polypoid AD in the small bowel that caused massive bleeding and eventually required surgical resection.
A 60-year-old male patient was admitted to our hospital with a 2-day history of exertional dyspnea. His vital signs were stable. There were no accompanying respiratory symptoms and abnormal breath sound on auscultation. His chest radiograph was normal; however, the laboratory findings showed severe anemia. The initial hemoglobin level was 6.3 g/dL, and the hematocrit level was 20.0% with a mean corpuscle volume of 98.0 fL. For 10 years, since he had undergone percutaneous coronary intervention for acute myocardial infarction, he had been taking aspirin and clopidogrel. However, there was no evidence of active bleeding or a bleeding tendency on history taking and physical examination. His blood test results were normal except for evidence of anemia, and echocardiography showed no other abnormal findings related to his cardiovascular condition.
An esophagogastroduodenoscopy found several erosions and small healing ulcers on the antrum. The colonoscopy result was normal. These findings were not sufficient to explain the severe anemia. For the small-bowel evaluation, capsule endoscopy was performed. It showed active bleeding from the proximal jejunum; however, the exact mucosal lesions were not determined (Fig. 1). Enteroscopy was planned for diagnostic and therapeutic purposes but could not be performed immediately because of the higher risk of bleeding related to the use of antiplatelet agents during the procedure. He stopped taking both aspirin and clopidogrel to prevent further bleeding. Computed tomography (CT) angiography revealed multifocal and small enhancing nodular lesions in the jejunum in the portal venous phase scan (Fig. 2). Mesenteric angiography showed dysplastic vessels accompanying early filling drainage at branches of the cecal and proximal jejunal arteries. Multiple slow-filling nodular stains in the small bowel were also detected. On the basis of these CT and angiographic findings, the small-bowel lesions were presumably diagnosed as unusual presentations of AD or hemangioma. During workup, packed red blood cells (RBCs) were transfused. The level of hemoglobin increased to 9.3 g/dL and remained stable. Dyspnea was relieved, with stable vital signs. He was discharged with the instruction to take aspirin only.
Three weeks later, he revisited our emergency room with massive hematochezia. The hemoglobin level was 5.7 g/dL, and the hematocrit level was 18.8% with the mean corpuscle volume of 92.6 fL. Accordingly, he stopped taking aspirin and received packed RBC transfusion again. CT angiography was performed, which showed multiple (>20) and small enhancing nodular lesions in the small bowel. Because there was no evidence of active arterial bleeding, embolization was considered not effective. Single-balloon enteroscopy was performed to evaluate the recurrent bleeding in the small bowel. It revealed multiple variable-sized bluish vascular lesions with a polypoid shape between the proximal and distal jejunum (Fig. 3). This finding was unusual for AD; thus, other vascular anomalies such as AVM or hemangioma were suspected first. Endoscopic treatment could be not implemented on the basis of the number and the location of lesions.
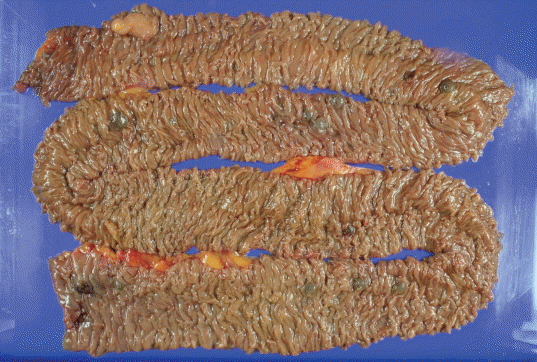
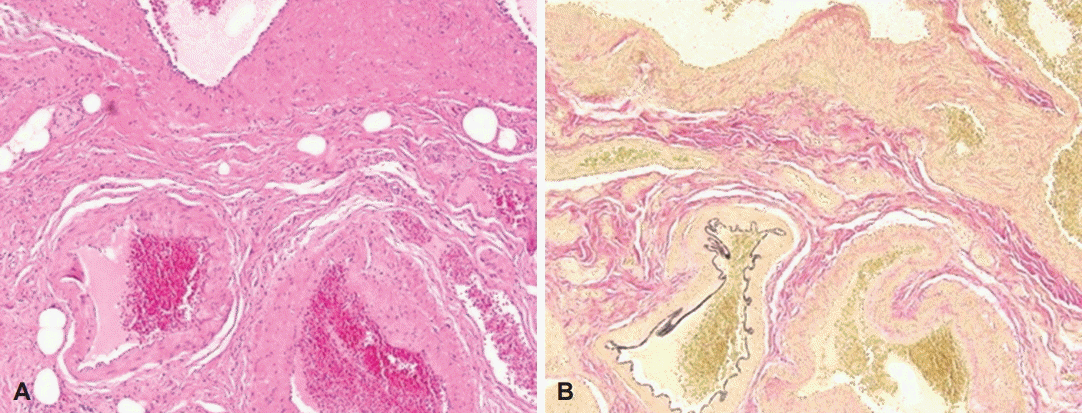
He continued to bleed and received an average of two units of packed RBCs per day. We decided to perform surgery for the small-bowel vascular lesions. During the surgery, enteroscopy was performed and the bleeding focus was found. However, there was no active bleeding. The small bowel was resected from 10 cm below the Treitz ligament, resulting in the resection of 120-cm length of the bowel. The specimen included 16 polypoid submucosal hematomas, with the largest measuring 1.5×1.3×0.8 cm (Fig. 4). During the pathologic diagnosis, hemangioma was initially suspected on the basis of the gross appearance. However, hematoxylin and eosin staining showed that the polypoid lesions consisted of ectatic vasculature with organizing thrombi in the submucosa, and elastin staining confirmed that the dilated and tortuous vasculatures were composed of arteries and veins with direct communication (Fig. 5). On the basis of these results, multiple polypoid AD was diagnosed. He fully recovered from surgery, and resumed taking both aspirin and clopidogrel. He has been doing well without further bleeding during the several months of follow-up.
AD is usually diagnosed on the basis of its typical endoscopic findings: bright red color, 5 to 10 mm diameter, and fern-like appearance with flare-radiating peripheral blood vessels [4]. AD usually has a flat or slightly raised morphology; a polypoid lesion is rare and therefore is not often reported. Of the 17 previous case studies on polypoid AD, most dealt with a single lesion with the exception of only one case involving multiple polypoid lesions. AD is considered a congenital error that occurs during the development of the embryonic vasculature. Moreover, a mechanical factor may play a role in its occurrence in some cases, with vigorous peristalsis or increased intraluminal pressure causing or increasing the shunting of blood into the submucosal arteriovenous system [5].
CT angiography is a potentially crucial noninvasive test because it could detect extravasation from AD [4]. Selective mesenteric angiography is also a useful diagnostic technique for AD, especially when patients experience massive bleeding that hinders an endoscopic approach. AD has three significant angiographic signs on mesenteric angiography. The earliest and the most common sign is an appearance of densely opacified, dilated, and slow-draining veins within the intestinal wall. As the lesion progresses, a vascular tuft can be found in the arterial phase scan. The late sign is an early filling of veins in the arterial phase scan, which signals an intensified arteriovenous communication throughout the angiodysplastic lesion [6]. Biopsy is not usually recommended because of the risk of bleeding during routine endoscopy; however, microscopic examinations of AD have revealed collections of dilated, tortuous arteries and veins in the submucosa [7].
Although the terms AD and AVM are used synonymously owing to their similar endoscopic and angiographic features, AD is histologically and clinically different from AVM. AVM is an interconnection of aberrant arteries and veins with thick hypertrophic walls. These lesions are usually congenital and tend to be found in younger patients. In contrast, AD has thin-walled submucosal vessels that are dilated, often lined by endothelium only. AD represents a degenerative process from intermittent or partial obstruction of submucosal veins, leading to capillary dilation, which results in arteriovenous communication [8,9]. Therefore, AD usually occurs in elderly patients and predominantly develops in the right colon [8].
Hemangioma in the small bowel is also very rare and can be divided into the capillary, cavernous, and mixed types. Endoscopy had revealed soft compressible submucosal lesions with a polypoid shape and bluish or deep-red color. Histologically, this lesion is distinct from that of AD or AVM. In the simplified classification of Mulliken and Glowacki, the important difference between hemangioma and vascular malformation lies in endothelial proliferation [10,11].
In this particular case, we detected multiple vascular lesions with a polypoid shape and bluish color on enteroscopy, and multiple nodular stains on CT. On the basis of these findings, small-bowel hemangioma was first suspected. However, there was no vascular proliferation lined by endothelial cells, suggesting AD in the pathological evaluation of our case.
The decision of whether to treat AD depends on the clinical situation. AD detected during endoscopy screening does not require treatment; however, patients with obscure occult or overt bleeding are candidates for AD treatment [12]. Endoscopic treatments such as argon plasma coagulation, electrocoagulation, and photocoagulation with Nd:YAG are the available modalities for AD treatment. For patients who cannot undergo endoscopic treatment, transcatheter angiography and embolization are encouraged as alternative treatments [13]. With the improvement of diagnostic and therapeutic modalities, the need to perform surgery has decreased [14]. However, our patient received surgery because the number and the location of the lesions—10 polypoid lesions between the proximal and distal jejunum—prevented endoscopic treatment. The repeated requirements for transfusion also influenced our decision to perform surgery. His preexisting and ongoing medications, such as aspirin and clopidogrel for coronary disease, also supported our decision. The comorbidities and clinical situation of each patient should be considered in the treatment of AD [15].
We report an extremely rare case of multiple polypoid AD that caused overt obscure GI bleeding. Endoscopic, radiologic, and histological evaluations all have an important role in the diagnosis and treatment of AD. Among the treatment modalities, surgery can be considered when endoscopic or angiographic treatment is not suitable, or when the patient has poor prognosis factors.
REFERENCES
1. Dray X, Camus M, Coelho J, Ozenne V, Pocard M, Marteau P. Treatment of gastrointestinal angiodysplasia and unmet needs. Dig Liver Dis. 2011; 43:515–522.

2. Lecleire S, Iwanicki-Caron I, Di-Fiore A, et al. Yield and impact of emergency capsule enteroscopy in severe obscure-overt gastrointestinal bleeding. Endoscopy. 2012; 44:337–342.

3. Regula J, Wronska E, Pachlewski J. Vascular lesions of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2008; 22:313–328.

4. Sharma R, Gorbien MJ. Angiodysplasia and lower gastrointestinal tract bleeding in elderly patients. Arch Intern Med. 1995; 155:807–812.

5. Rodriguez-Jurado R, Morales SS. Polypoid arteriovenous malformation in the jejunum of a child that mimics intussusception. J Pediatr Surg. 2010; 45:E9–E12.

6. Dodda G, Trotman BW. Gastrointestinal angiodysplasia. J Assoc Acad Minor Phys. 1997; 8:16–19.
7. Sami SS, Al-Araji SA, Ragunath K. Review article: gastrointestinal angiodysplasia: pathogenesis, diagnosis and management. Aliment Pharmacol Ther. 2014; 39:15–34.
8. Rizvi AZ, Kaufman JA, Smith P, Silen ML. Solitary arteriovenous malformation of the small intestine. J Am Coll Surg. 2005; 200:808–809.

10. Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982; 69:412–422.
11. Burrows PE, Mulliken JB, Fellows KE, Strand RD. Childhood hemangiomas and vascular malformations: angiographic differentiation. AJR Am J Roentgenol. 1983; 141:483–488.

12. Foutch PG, Rex DK, Lieberman DA. Prevalence and natural history of colonic angiodysplasia among healthy asymptomatic people. Am J Gastroenterol. 1995; 90:564–567.
13. Funaki B. Endovascular intervention for the treatment of acute arterial gastrointestinal hemorrhage. Gastroenterol Clin North Am. 2002; 31:701–713.

Fig. 1.
(A-D) Capsule endoscopy image showing active bleeding from the proximal jejunum, the exact mucosal lesions could not be detected (arrows).
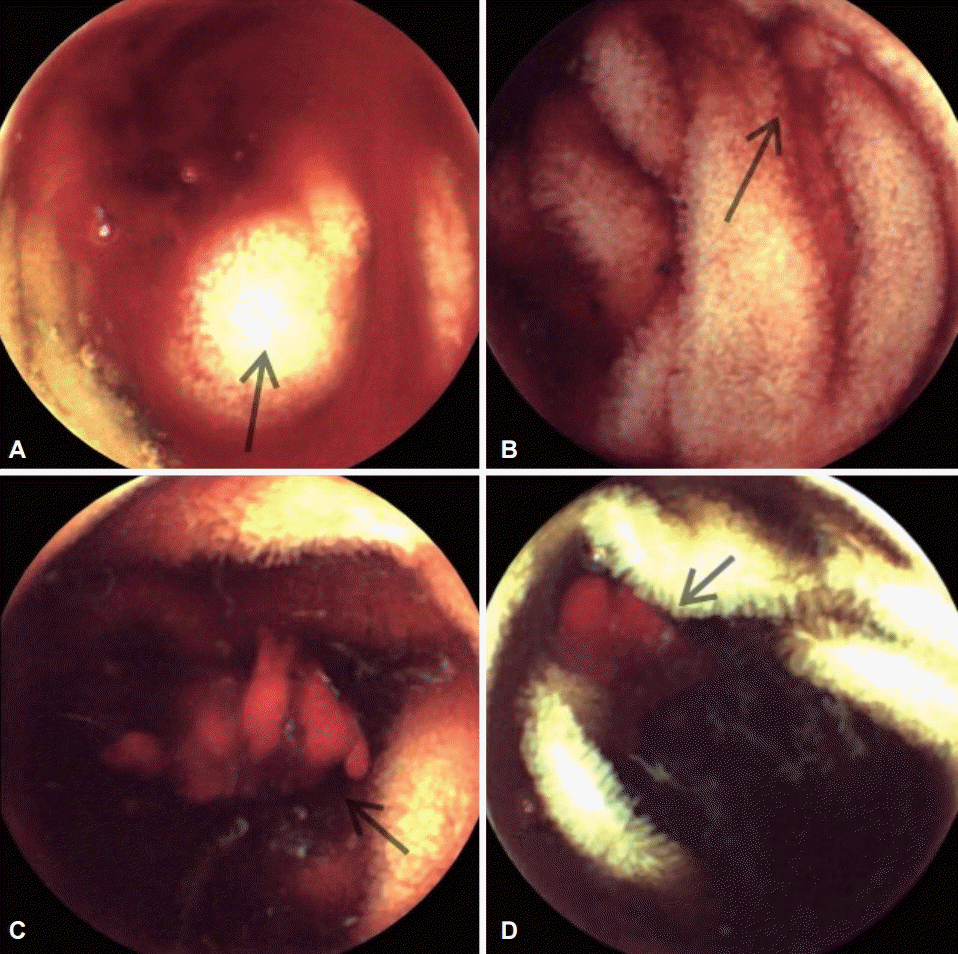
Fig. 2.
(A-D) Computed tomography angiography scan showing multifocal small enhancing nodular lesions in the jejunum (arrows).
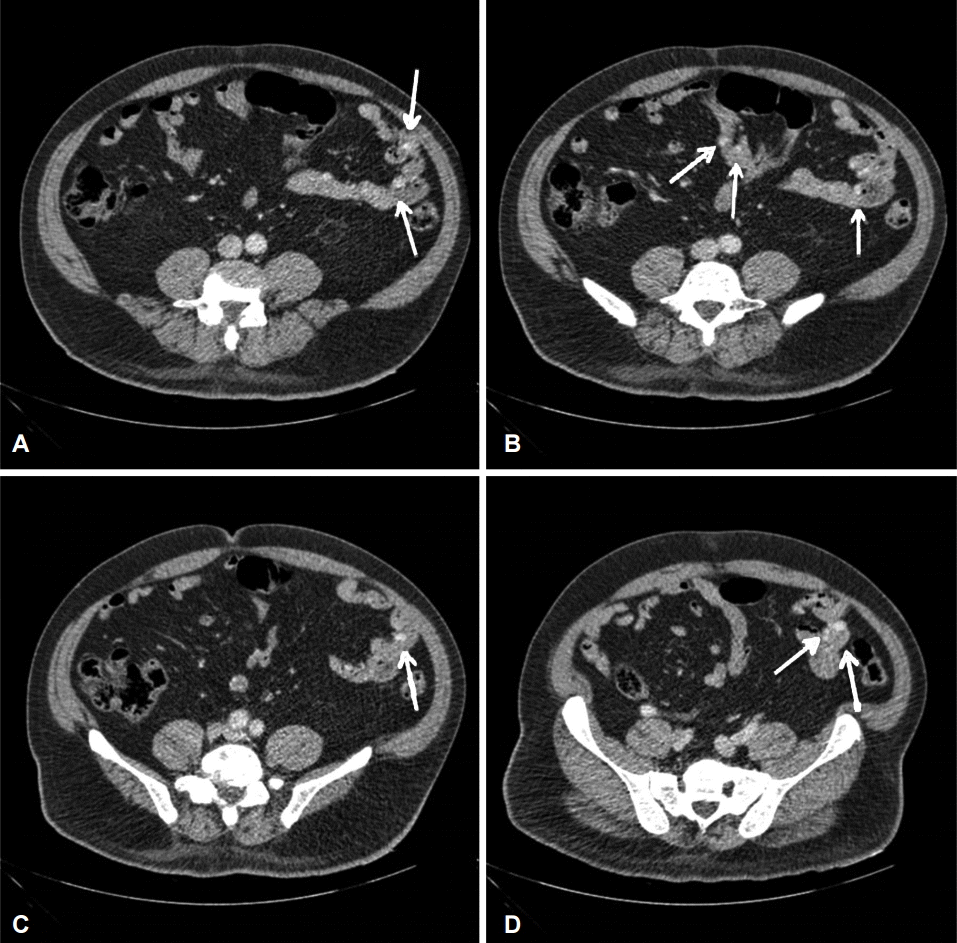
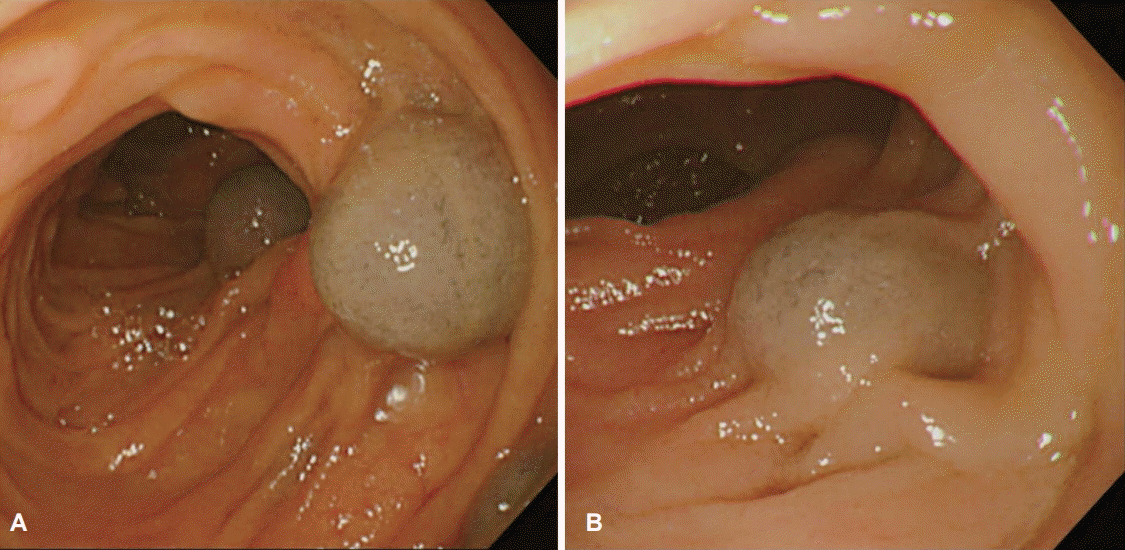
Fig. 3.
Single-balloon enterocopy image show multiple, variabl-sized, bluish vascular lesions with a polypoid shape between the proximal (A) and distal (B) jejunum.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download