Abstract
Background/Aims:
The use of moderate to deep sedation for gastrointestinal endoscopic procedures has increased in Europe considerably. Because this level of sedation is a risky medical procedure, a number of international guidelines have been developed. This survey aims to review if, and if so which, quality aspects have been included in new sedation practices when compared to traditional uncontrolled sedation practices.
Methods:
A questionnaire was sent to the National Associations of Nurse Anesthetists in Europe and the National Delegates of the European Section and Board of Anaesthesiology from January 2012 to August 2012.
Results:
Huge variation in practices for moderate to deep sedation were identified between and within European countries in terms of safety, type of practitioners, responsibilities, monitoring, informed consent, patient satisfaction, complication registration, and training requirements. Seventy-five percent of respondents were not familiar with international sedation guidelines. Safe sedation practices (mainly propofol-based moderate to deep sedation) are rapidly gaining popularity.
Conclusions:
The risky medical procedure of moderate to deep sedation has become common practice for gastrointestinal endoscopy. Safe sedation practices requiring adequate selection of patients, adequate monitoring, training of sedation practitioners, and adequate after-care, are gaining attention in a field that is in transition from uncontrolled sedation care to controlled sedation care.
Since Basil Hirschowitz [1,2] invented a useful flexible endoscope in 1958, which was further developed later [3], gastrointestinal (GI) endoscopy has grown from a simple diagnostic procedure to complex time-consuming diagnostic and therapeutic invasive interventions. These procedures may be painful and unpleasant to undergo. Although sedation for these procedures is traditionally part of the quality and safety domain of the specialty of anesthesia, the capacity of anesthesiologists is too limited to meet the increasing demand for sedation care in most countries, causing the development of solutions where quality and patient safety have not been the primary drivers.
Moderate-to-deep procedural sedation and analgesia by long-acting sedative drugs has been increasingly replaced by a combination of propofol or benzodiazepines (midazolam) and/or a short-acting opioid for use in patients undergoing GI endoscopic procedures outside the operation room area. High-quality sedation reduces anxiety and discomfort for the patient and improves the quality of the examination or therapy during these procedures. Moderate-to-deep sedation procedures are potentially risky and have to be carried out by trained professionals under specific safety conditions in order to achieve a high level of quality, safety, and comfort.
The present study was conducted to evaluate how far controlled sedation care (CSC) practices have been implemented when compared to traditional uncontrolled sedation care (USC) practices during GI endoscopy (including endoscopic retrograde cholangiopancreatography [ERCP], colonoscopy, and esophagogastroduodenoscopy), following the publication of the 2010 European [4,5] guidelines for moderate and deep sedation.
We carried out an online survey (Appendix 1) of the National Associations of Nurse Anaesthetists in Europe and the National Delegates of the European Section and Board of Anaesthesiology.
In contrast to light sedation using small doses of midazolam (1 to 3 mg), traditional USC is defined as a moderate-to-deep sedation procedure [6-8] (usually benzodiazepines with or without opioids) carried out by a person [9] who may have other responsibilities during the procedure. A characteristic of USC is the use of more or less fixed-dose protocols for sedatives and/or opioids and the use of a variety of patient monitoring methods. In contrast, CSC is defined as moderate-to-deep sedation (usually using propofol with or without opioids). CSC is characterized by formal screening of the health status of the patient and is carried out by a trained and certified (MD or nurse) sedation practitioner, whose sole responsibility is the execution of the sedation procedure and personal observation and standardized monitoring (e.g., pulse oximetry, electrocardiogram [ECG], non-invasive blood pressure measurements [NIBP]) of the patient during the procedure, the recovery, and discharge according to formal discharge and after-care criteria.
Light, moderate, and deep sedation guidelines were defined according to international definitions [10,11] and a 5-chapter, 21-item questionnaire (Appendix 1) was developed for the study in accordance with a collaborative effort from representatives of the European Society of Gastrointestinal Endoscopy (ESGE), the European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology. Participants were asked to answer questions pertaining to comparing USC versus CSC during GI endoscopy against the background of the implementation of recent sedation guidelines. Questions were formulated about demographics, sedation technique, the sedation practitioner, patient monitoring, complications, training, informed consent, and patient satisfaction. Patient satisfaction quality indicators have been described as amnesia, the patient´s opinion, no pain after the procedure, quick recovery, and patient comfort. Respondents were requested to indicate the content of skills training programs for the sedation officer for USC and CSC. The international online linked survey was performed from January 2012 until August 2012. The electronic mail addresses of the National Associations of Nurse Anaesthetists in Europe [12] and the European Section and Board of Anaesthesiology [13] were provided by both organizations. Efforts to increase the response rate were carried out by sending reminders twice by electronic mail.
A total of 68 surveys (Appendix 1) were sent to multiple addresses in Europe by electronic mail: 18 surveys to the National Associations of Nurse Anaesthetists in Europe (Austria, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Ireland, Italy, Luxembourg, The Netherlands, Norway, Poland, Slovak Republic, Spain, Sweden, Switzerland, and UK) and 50 surveys to the national delegates of the European Section and Board of Anaesthesiology. Our data were primarily obtained from anesthesiology-associated respondents.
Thirty-three (response rate 48.5%) contributors completed the survey. Respondents were from Spain, Italy, The Netherlands, Germany, Austria, Poland, France, Switzerland, Belgium, Bulgaria, Czech Republic, England, Luxembourg, Norway, Portugal, and Sweden. Two contributions did not mention their country of origin.
Seven countries (Belgium, Czech Republic, England, Italy, Norway, The Netherlands, and Sweden) indicated that 50% or more patients were served by USC for GI endoscopy procedures, usually using a combination of midazolam and a short-acting opioid. Eight countries (Austria, Bulgaria, France, Germany, Poland, Portugal, Spain, and Switzerland) indicated that more than half of the patients were served by CSC care for GI endoscopy procedures, usually with a combination of propofol and/or a short-acting opioid (Table 1).
The person who performed the sedation procedure differed from country to country, within countries, and within hospitals. Both anesthesiologists (MDs), nurse anesthetists, endoscopists (MDs), endoscopy assistants being supported or supervised by endoscopists (MDs), physician assistants, and other health care personnel, being trained in the art of sedation or not formally trained, provided moderate-to-deep sedation. The anesthesiology department is ultimately medically responsible [14] for moderate-to-deep sedation except in Sweden. Sedation is restricted to anesthesiologists in Bulgaria, the Czech Republic, Luxembourg, and Portugal (Table 2).
Survey respondents providing CSC during GI endoscopy indicated that routinely one or more vital signs in all patients were monitored. During USC, pulse oximetry was frequently monitored. The respondents indicated routine pulse oximetry and heart rate (100%) evaluation, NIBP (94%), ECG (59%), and capnography (47%) or a combination during CSC procedures (Table 3). Routine monitoring in the recovery room after a CSC procedure consisted of pulse oximetry (100%), heart rate and NIBP (94%), ECG (53%), and capnography (24%), or a combination, in Europe (Table 4).
In both the USC and CSC group, informed consent for the sedation procedure was obtained from 65% of the patients.
Survey respondents were asked about the “24 hours a day, 7 days a week” sedation service for GI endoscopy. Forty-four percent of the respondents reported that such a service was available. In other hospitals, urgent endoscopic procedures out of office hours were performed under general anesthesia.
Adherence to the European International guidelines for sedation was variable. About 25% of the respondents indicated they adhered to international sedation guidelines for moderate-to-deep sedation. Seventy-five percent of respondents indicated they were not familiar with these guidelines.
The majority (60%) of the respondents reported they had organized a patient complication registration data system when CSC was performed. Those who did not register complications cited the following reasons: no database available, insufficient staff to register complications, and no protocol to register complications.
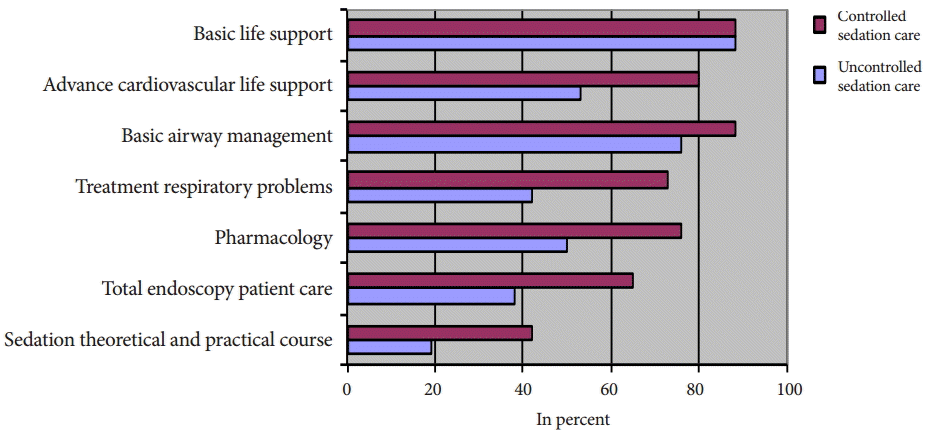
In both groups, the responsible sedationists for USC and CSC had been trained in basic life support (88%). Slightly more than half (53%) of sedationists in the USC group had undergone advanced cardiac life support training whereas 80% of the CSC group had done so. Basic airway management training had been taught to 76% of USC practitioners, in contrast to 88% of the CSC sedation nurses. For further skills training program data see Fig. 1.
The technical advances in diagnostic and therapeutic procedures, particularly in gastroenterology [15], have caused an exponential increase in these procedures in the last few decades. These procedures frequently require cooperation from the patient, are not pleasant to undergo, and patients require support and comfort in order to cooperate. Moderate-to-deep sedation is able to meet these requirements.
The best methods for moderate-to-deep sedation during GI endoscopy are still a matter of debate, depending on the quality indicators considered (e.g., patient comfort, safety, working conditions for the endoscopist, budget impact). Taking into account the widely known shortages of medical anesthesia personnel in Europe available to provide sedation care, alternative solutions to meet the sharply rising need for adequate sedation have been introduced in many European countries, not always considering quality and safety but instead efficiency and efficacy as starting points or as primary drivers. This has caused unnecessary morbidity and mortality related to sedation [16,17]. Moderate-to-deep sedation is a risky medical procedure, even when performed by trained and qualified sedation staff [18]. Therefore, considerable attention must be focused on the adequate selection of patients and close monitoring of vital parameters, particularly when performing long-lasting interventions and emergency procedures.
Apart from the endoscopist, a well-trained sedation professional should be responsible for clinical and instrumental monitoring of the patient during GI endoscopy, as recommended at the International Sedation Endoscopy Workshop in 2009 [19-21]. In Europe in particular the debate on propofol-based sedation is strongly influenced by different legislation between countries and reimbursement matters and unfortunately not always by quality arguments [22].
The use of propofol for moderate-to-deep sedation by non-anesthesiologists and by non-medical health care personnel is a matter of debate in many countries and various arguments are used [23]. The properties of propofol as a hypnotic in anesthesia are well known, and vast experience has been gained in handling the side effects of propofol overdosing. The use of propofol for moderate-to-deep sedation; however, is a titration technique, which is essentially different from its use as a hypnotic and requires new and different skills from the sedation practitioner as compared to propofol when used for general anesthesia. Fortunately, numerous examples are available that show that non-anesthesiologist sedation practitioners, when properly trained, can handle propofol as a sedative appropriately and safely [24,25].
Additional risk factors caused by the comorbidity of the patients and the nature of the endoscopic procedure play important roles in determining whether the support of an anesthesia team is needed for moderate-to-deep sedation. We found in our survey a huge variability in sedation practices, which could not be attributed to differences of purely medical origin. The conclusion must be that factors other than quality or patient safety are responsible for the variation of practices. Since moderate-to-deep sedation is a risky procedure for high-risk patients, quality and safety should be primary concerns.
Our survey among the National Associations of Nurse Anaesthetists in Europe and among the National Delegates of the European Section and Board of Anaesthesiology shows that, in particular, the debate on propofol-based sedation is strongly influenced by different legislation between countries and reimbursement matters and unfortunately not always by quality arguments. Our survey clearly shows the wide variability of practice for moderate-to-deep sedation, of the variable skills of sedation practitioners, of the final medical responsibilities, and of quality standards of care for a procedure with an established morbidity and mortality. This situation is typically characteristic for a transition period, where first practical solutions have been devised for a rapidly increasing need for moderate-to-deep sedation, to be followed by quality measures to make sedation safe. The need for quality in sedation means that increasing numbers of health care authorities are taking steps to control implementation processes for sedation and maintain quality standards by law [26]. Sedation as a risky medical procedure requires adherence to medical protocols designed around the comfort and safety for patients, the development of training programs for sedation practitioners, adequate screening of patients, adequate and safe monitoring, and appropriate after-care of patients.
Moreover, it becomes clear that the general public increasingly refuses to accept medical procedures that are unnecessarily uncomfortable or painful [27,28]. Generally speaking, according to the data of our survey, CSC care was slightly more prevalent than USC in highly complex interventional GI endoscopic procedures such as ERCP and others. The time needed to treat these patients efficiently tends to be much longer than the time required for conventional GI endoscopy procedures such as colonoscopy. In these complicated cases, sedation is therefore carried out intending to induce a state of deep sedation [29]. At this consciousness level, patients may respond to repeated or painful stimuli, and spontaneous respiration can be unstable and insufficient. The risk of developing serious complications is considerable if strict quality and safety measures are not met. Unfortunately, CSC 24/7 service is limited in availability. The reasons for not providing a “24 hours a day, 7 days a week” CSC care involved no demand for it outside of normal working hours, sedation service is only available for elective cases, and no available staff. Overall, patient satisfaction was monitored in 14% of the cases according to the findings of Staff et al. [30].
Taking into account the variability of medico-legal rules and legal restrictions in many European countries, the responsibility for sedation procedures also varies widely and lies with anesthesiologists, nurse anesthetists, gastroenterologists, endoscopic assistants, physician assistants, emergency physicians, sedation practitioners, and others. Other differences may be caused by factors such as the organization of health care, the availability of training programs for sedation professionals, the anesthesiologist work force, and reimbursement. Factors such as available equipment and expectations and demands from the patients might have played a role as well. An ESGE [31] survey amongst its members approximately 6 years before our study reported that in about 50% of ESGE-related countries, less than 25% of patients were sedated for routine diagnostic upper GI endoscopy. Our 2012 study shows that the application of CSC in gastroendoscopy has increased considerably when compared to the ESGE 2006 data, although the methodology used was different.
An encouraging observation from our survey is that instrumental monitoring seems to be applied more abundantly than in 2006, contributing to patient safety [32]. This is probably caused by the realization of the risks associated with moderate-to-deep sedation [33-35]. It is imperative to develop uniform definitions of sedation and complications. This is important for the discussion to make sedation procedures safe, comfortable, and of high quality.
However, our study has some important limitations with restrictive consequences for our conclusions. Our data were basically retrieved from anesthesiology-associated respondents. Data gathered from gastroenterologists may produce a different image. However, true national data on sedation are virtually impossible to uncover, because databases on sedation procedures are lacking in virtually all European countries.
In conclusion, in this survey conducted of anesthesia professionals, we identified a considerable variability of the practice of sedation in European countries. Notwithstanding the presence of international guidelines, the lack of formal implementation processes has limited the development of uniform policies of sedation, obstructing comparative scientific research into quality and outcomes of sedation [36,37]. For a risky medical procedure such as moderate-to-deep sedation further improvement of quality by harmonization of practices will contribute to quality, patient safety, and comfort. The international guidelines were translated into medical practice to a very limited extent. Through this study, it becomes clear that there are many changes taking place in sedation practices in Europe, but much remains to be done to ensure maximum safety of the sedated patient.
ACKNOWLEDGMENTS
A kind word of thanks to the European National Associations of Nurse Anaesthetists and the European Section and Board of Anaesthesiology for their willingness to fill in our survey.
REFERENCES
1. Hirschowitz BI, Curtiss LE, Peters CW, Pollard HM. Demonstration of a new gastroscope, the fiberscope. Gastroenterology. 1958; 35:50.

2. Hirschowitz BI. Endoscopy: 40 years since fiber optics. Any light at the end of the tunnel? Dig Surg. 2000; 17:115–117.
3. Garborg KK, Løberg M, Matre J, et al. Reduced pain during screening colonoscopy with an ultrathin colonoscope: a randomized controlled trial. Endoscopy. 2012; 44:740–746.

4. Dumonceau JM, Riphaus A, Aparicio JR, et al. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: non-anaesthesiologist administration of propofol for GI endoscopy. Eur J Anaesthesiol. 2010; 27:1016–1030.

5. Dumonceau JM, Riphaus A, Aparicio JR, et al. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: non-anesthesiologist administration of propofol for GI endoscopy. Endoscopy. 2010; 42:960–974.

6. American Society of Anesthesiologists. Distinguishing monitored anesthesia care (“MAC”) from moderate sedation/analgesia (conscious sedation) [Internet]. Parkridge: American Society of Anesthesiologists;c1995. [cited 2015 Dec 30]. Available from: file:///C:/Users/USER/Downloads/distinguishing-monitored-anesthesia-care-from-moderate-sedation-analgesia%20(1).pdf.
7. Joint Commission Resources Organizations. New definitions, revised standards address the continuum of sedation and anesthesia. Jt Comm Perspect. 2000; 20:10.
9. Eberl S, Polderman JA, Preckel B, Kalkman CJ, Fockens P, Hollmann MW. Is “really conscious” sedation with solely an opioid an alternative to every day used sedation regimes for colonoscopies in a teaching hospital? Midazolam/fentanyl, propofol/alfentanil, or alfentanil only for colonoscopy: a randomized trial. Tech Coloproctol. 2014; 18:745–752.

10. American Society of Anesthesiologists. Continuum of depth of sedation: definition of general anaesthesia and levels of sedation/analgesia [Internet]. Parkridge: American Society of Anesthesiologists;c1995. [cited 2015 Dec 30]. Available from: http://bit.ly/11iGIax.
11. Waring JP, Baron TH, Hirota WK, et al. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc. 2003; 58:317–322.

12. Meeusen V, van Zundert A, Hoekman J, Kumar C, Rawal N, Knape H. Composition of the anaesthesia team: a European survey. Eur J Anaesthesiol. 2010; 27:773–779.

13. EBA UEMS. The European Section and Board of Anesthesiology is the Anesthesiology branch of UEMS (European Union Medical Specialities) dealing primarily with Anesthesia and Resuscitation, as well as Intensive Care, Emergency and Pain Medicine [Internet]. Brussels: EBA UEMS;c2015. [cited 2015 Dec 30]. Available from: http://www.eba-uems.eu.
14. American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002; 96:1004–1017.
15. McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc. 2008; 67:910–923.

16. Nayar DS, Guthrie WG, Goodman A, et al. Comparison of propofol deep sedation versus moderate sedation during endosonography. Dig Dis Sci. 2010; 55:2537–2544.

17. Zippi M, Traversa G, De Felici I, et al. Sedation with propofol in endoscopic retrograde cholangiopancreatography: personal experience. Clin Ter. 2008; 159:19–22.
18. Patel S, Vargo JJ, Khandwala F, et al. Deep sedation occurs frequently during elective endoscopy with meperidine and midazolam. Am J Gastroenterol. 2005; 100:2689–2695.

19. Schilling D, Rosenbaum A, Schweizer S, Richter H, Rumstadt B. Sedation with propofol for interventional endoscopy by trained nurses in high-risk octogenarians: a prospective, randomized, controlled study. Endoscopy. 2009; 41:295–298.

20. Rex DK, Deenadayalu VP, Eid E, et al. Endoscopist-directed administration of propofol: a worldwide safety experience. Gastroenterology. 2009; 137:1229–1237.

21. Müller M, Wehrmann T, Eckardt AJ. Prospective evaluation of the routine use of a nasopharyngeal airway (Wendl Tube) during endoscopic propofol-based sedation. Digestion. 2014; 89:247–252.

22. Perel A. Non-anaesthesiologists should not be allowed to administer propofol for procedural sedation: a Consensus Statement of 21 European National Societies of Anaesthesia. Eur J Anaesthesiol. 2011; 28:580–584.
23. Külling D, Orlandi M, Inauen W. Propofol sedation during endoscopic procedures: how much staff and monitoring are necessary? Gastrointest Endosc. 2007; 66:443–449.

24. Rex DK, Overley C, Kinser K, et al. Safety of propofol administered by registered nurses with gastroenterologist supervision in 2000 endoscopic cases. Am J Gastroenterol. 2002; 97:1159–1163.

25. Werner C, Smith A, Van Aken H. Guidelines on non-anaesthesiologist administration of propofol for gastrointestinal endoscopy: a double-edged sword. Eur J Anaesthesiol. 2011; 28:553–555.
26. Axon AE. The use of propofol by gastroenterologists: medico-legal issues. Digestion. 2010; 82:110–112.

27. von Delius S, Hollweck R, Schmid RM, Frimberger E. Midazolam-pain, but one cannot remember it: a survey among Southern German endoscopists. Eur J Gastroenterol Hepatol. 2007; 19:465–470.

28. van Gelder RE, Birnie E, Florie J, et al. CT colonography and colonoscopy: assessment of patient preference in a 5-week follow-up study. Radiology. 2004; 233:328–337.

29. Garewal D, Vele L, Waikar P. Anaesthetic considerations for endoscopic retrograde cholangio-pancreatography procedures. Curr Opin Anaesthesiol. 2013; 26:475–480.

30. Staff DM, Saeian K, Rochling F, et al. Does open access endoscopy close the door to an adequately informed patient? Gastrointest Endosc. 2000; 52:212–217.

31. Ladas SD, Aabakken L, Rey JF, et al. Use of sedation for routine diagnostic upper gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy Survey of National Endoscopy Society Members. Digestion. 2006; 74:69–77.

32. Travis AC, Pievsky D, Saltzman JR. Endoscopy in the elderly. Am J Gastroenterol. 2012; 107:1495–1501.

33. Sharma VK, Nguyen CC, Crowell MD, Lieberman DA, de Garmo P, Fleischer DE. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc. 2007; 66:27–34.

34. Standards of Practice Committee, Lichtenstein DR, Jagannath S, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008; 68:205–216.

35. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.

Table 1.
Patients Served by USC or CSC Care
Table 2.
Sedation Practitioner Healthcare Professional Performing Controlled Sedation Care during Gastrointestinal Endoscopy
| Country | Anesthesiologist (MD) | Endoscopist (MD) | Endoscopist nurse | Nurse administered propofol sedation | Non-anesthesiologist | Endoscopy assistant (MD) | Nurse anesthetist | Sedation practitioner |
|---|---|---|---|---|---|---|---|---|
| Austria | × | |||||||
| Belgium | × | |||||||
| Bulgaria | ★a) | |||||||
| Czech Republic | ★a) | |||||||
| France | × | |||||||
| Germany | × | |||||||
| Great Britain | × | × | ||||||
| Italy | × | × | × | |||||
| Luxembourg | ★a) | |||||||
| Norway | × | |||||||
| Poland | × | |||||||
| Portugal | ★a) | |||||||
| Spain | × | × | × | |||||
| The Netherlands | × | × | ||||||
| Switzerland | × | × | × | |||||
| Sweden | × |
Table 3.
Routine Patient Controlled Sedation Care Monitoring during Gastrointestinal Endoscopy
Table 4.
Monitoring during Recovery after Controlled Sedation Care Gastrointestinal Endoscopy




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download