Abstract
Technological advances have rapidly expanded the therapeutic potential of endoscopic ultrasound (EUS). Innovations in stent technology; directed adjunctive therapy for pancreatic tumors, including radiofrequency ablation and fiducial marker placement; advanced imaging modalities, including needle-based confocal laser endomicroscopy; and new echoendoscopes, such as the forward-viewing linear echoendoscope, are emerging as safe and effective tools and devices for providing a broad range of treatments and therapies previously not thought possible. In this review, we summarize and discuss the new echoendoscopes, accessories, and stents for interventional EUS and highlight the recent literature on technical and therapeutic efficacy. The therapeutic role and indications for EUS are rapidly evolving well beyond its current limits as new EUS-specific designed tools are designed, and ultimately, should help achieve the goal of improving patient outcomes.
Since its inception in the 1980s, endoscopic ultrasound (EUS) has continued to evolve. Over the past decade, various new tools have been developed to expand the therapeutic potential of EUS. EUS can now provide access to various fluid collections and to different portions of the gastrointestinal (GI) tract, as well as therapy (either directly or help guide it) for tumors and enhanced imaging capabilities to assist diagnosis. These new tools and devices can then be delivered through new echoendoscopes with forward-viewing capabilities that may facilitate interventional EUS procedures.
In the past, EUS-guided transluminal drainage of pancreatic fluid collections (PFCs), as well as obstructed biliary and pancreatic ducts, were performed by using plastic and metal stents originally designed for use with endoscopic retrograde cholangiopancreatography (ERCP). However, there are major drawbacks to using these types of stents, which do not allow for lumen apposition to anchor the stent in position, are often too long, and cannot properly seal the apposing lumens. Therefore, complications encountered can include leakage, perforation, tissue damage, and migration. To overcome these issues, lumen-apposing metal stents (LAMS) were designed specifically for transluminal drainage and appear to have the advantage of easy deployment and offer the ability to perform endoscopic debridement through them, with low rates of migration and other complications. A few other short-flanged metal stents have also been produced, but are not widely available, with limited published data on their efficacy. Images (Figs. 1, 2) and descriptions of the stents described below were recently published in Weilert et al.’s review [1].
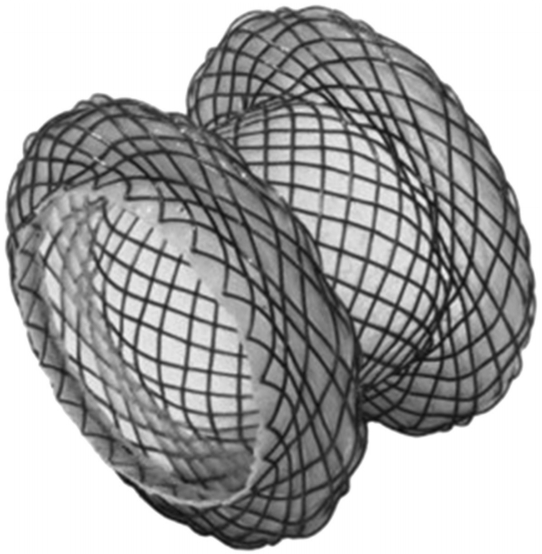
Binmoeller and Shah [2] were the first to publish an article on the creation of LAMS for endoscopic drainage in 2011. The Axios stent (Boston Scientific, Natick, MA, USA) consists of double-walled flanges with diameters that are double the stent lumen (dumbbell shape) and hold the tissue walls in apposition (Fig. 1). In the United States, the currently available stent diameters are 10 and 15 mm. The stent design consists of nitinol wire, which is fully coated to prevent leakage and allow removal. The large flanges and a short stent length of 10 mm are designed to distribute pressure evenly on the lumenal wall and allow anastomosis creation. More recently, the 10-Fr Axios stent delivery system has been modified to include a cautery tip to facilitate puncture through the GI tract wall (HotAxios; Boston Scientific) and obviate the need for dilation to deploy the stent. This stent has only recently been approved for use in Europe and the United States.
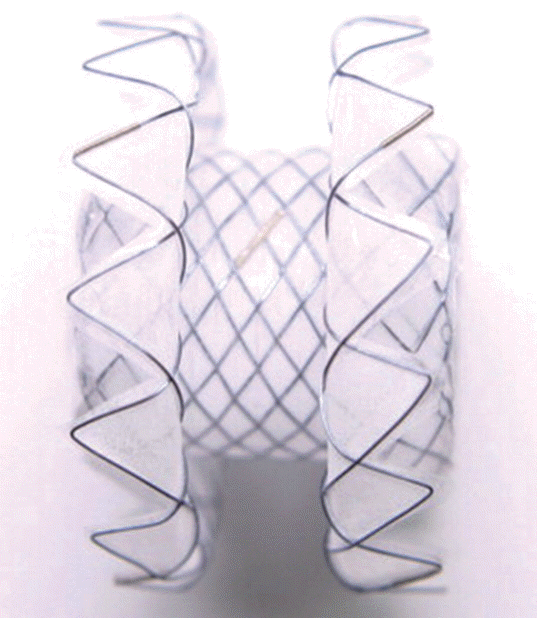
The Niti-S Spaxus stent (Taewoong Medical Co. Ltd., Goyang, Korea) was first described by Moon et al. [3], and is another LAMS, similar to the Axios stent. It is also made of nitinol wire, is fully covered, delivered with a 10-Fr system, and has large-diameter flanges that transmit even pressure on the lumen wall [3]. The diameter of the flanges at both ends is 25 mm (Fig. 2). One unique feature of the Spaxus stent is that the flanges fold back once fully deployed, which is proposed to enhance lumen apposition and prevent migration. The available diameters created include 8, 10, and 16 mm, with a length of 20 mm. However, the distance between the flanges is a mere 5 mm after they fold back during deployment. This stent is not commercially available currently.
There have been multiple studies to date examining the safety and efficacy of LAMS (most of the available prospective data pertain to the Axios stent) when used to drain PFCs [4]. Itoi et al. [5] first reported the use of the Axios stent for symptomatic pancreatic pseudocysts in 2012 (15 patients). All stents were successfully deployed without complications (median time of removal, 15 days), with only one stent migration reported. All patients had complete resolution of their collections following the initial procedure and no recurrence during the year of follow-up. In addition, they demonstrated the safety and feasibility of performing pancreatic necrosectomy through the deployed Axios stent [5].
Gornals et al. [6] published a study in 2013 comparing Axios (n=9) with plastic double pigtail stents (n=10). The technical success rate for Axios was 88.8%, with one reported delivery system failure. All patients achieved complete PFC resolution following the initial procedure (stent retrieval 33±40 days). However, there was a pseudocyst recurrence 1 month after stent removal, and a pneumothorax was seen after transesophageal drainage. Compared to plastic pigtail stents, the Axios stent showed similar technical and clinical success. However, the patients with double pigtail stents had higher rates of complications, recurrences, and stent migrations.
In 2015, Shah et al. [7] published a prospective, multicenter study of 33 patients that showed that Axios stents were placed successfully in 91% of subjects with PFCs and had a 93% resolution rate. The complications (15%) were abdominal pain (n=3), fever requiring hospitalization (n=1), back pain (n=1), access-site infection (n=1), and stent migration/dislodgement (n=1).
EUS-guided gallbladder drainage (EUS-GBD) is a relatively new approach with limited published data. One retrospective study evaluated 15 non-surgical candidates who underwent EUS-GBD with LAMS to decompress the gallbladder (seven patients with calculous cholecystitis, four with acalculous cholecystitis, two with biliary obstruction, one with gallbladder hydrops, and one with symptomatic cholelithiasis) [8]. All had refused percutaneous drainage. Technical success was reported as 93%, with only one patient developing post-procedure fever. However, major study limitations were a small sample size and a retrospective design.
A larger, multicenter prospective trial examined the safety of LAMS for EUS-GBD in 30 patients [9]. Technical success was achieved in 90% of the patients, and the clinical success rate was 96%. LAMS removal was performed in 15 of 30 patients (50%) after a mean of 91 days (SD±24 days). In 15 patients (50%), no LAMS removal was performed because of death (n=5), significant tissue overgrowth (n=2), or other causes (n=8). The authors reported a high rate (50%) of serious adverse events in 15 patients, which included stent occlusion with recurrent cholecystitis.
Prior studies on EUS-GBD utilized standard LAMS; however, there has been a report on utilizing the modified HotAxios stent with the cautery capability on the tip to aid passage of the delivery system through the GI tract lumen and into the gallbladder without the need for tract dilation prior to stent deployment [10].
Itoi et al. [11] was the first to describe the technique of using LAMS for EUS-guided gastrojejunostomy in 2013. Itoi’s group has lead the research on this technique and has published additional results more recently. This technique involves utilizing a specially created double-balloon enteric tube (Tokyo Medical University type, Create Medic, Yokohama, Japan) to help stabilize the small bowel adjacent to the stomach in the area of the puncture and LAMS placement.
Itoi et al. [12] conducted an animal study examining the use of different types of LAMS (Axios, HotAxios, and Spaxus) for creating a gastrojejunostomy. Survival experiments were conducted for 1 month and showed that animals had normal eating behavior and no signs of infection. On follow-up endoscopy, the LAMS appeared patent, and once removed, a mature stoma was noted that allowed for easy scope passage from the stomach into the jejunum.
Finally, Itoi et al. [13] published their results from the first prospective study on EUS-guided double-balloon occluded gastrojejunostomy bypass using LAMS in 20 patients in 2015. The technical success rate was 90%; however, in the two failed cases, there was misdeployment of the distal flange, resulting in pneumoperitoneum and perforation.
Due to the exclusion of the distal stomach and proximal duodenum in Roux-en-Y gastric bypass, the ampulla cannot be accessed via standard ERCP procedure. Although deep enteroscopy can be performed, the success rates are variable. Recently, the use of LAMS to provide access to the excluded GI tract lumen for passage of the duodenoscope for ERCP has been proposed by Kedia et al. [14] EUS-guided creation of a gastrogastric or jejunogastric fistula via the placement of LAMS was successful in all five cases studied (100%). The ability to perform ERCP with LAMS during the index procedure was successful in three of five cases (60%). Two LAMS dislodgments required restenting. No major adverse events were observed. A major limitation of this study is the extremely small sample size of only five patients, and larger studies are needed to further assess safety, efficacy, and long term follow-up, including durability of fistula closure and any effects on weight gain.
There have been two types of radiofrequency ablation (RFA) probes developed and studied to date, a probe that passes through a needle (Habib; EMcision, London, UK) and a needle with specially designed tip (VIVA RF generator; STARmed, Goyang, Korea). The Habib EUS-RFA probe is a novel monopolar catheter that can be used to cauterize and coagulate tissue. This probe consists of a 1-Fr wire (0.33 mm diameter) that has a working length of 220 cm and can be passed through a standard 19- or 22-gauge EUS needle. Radiofrequency power is applied to the 20-mm electrode at the end of the wire to cauterize or coagulate tissue. The Habib EUS-RFA probe is approved for use in Europe and the United States currently. It has been studied as a potential treatment for pancreatic cystic neoplasms and neuroendocrine tumors [15]. A prospective study examined the use of EUS-guided RFA in eight patients with pancreatic lesions (six cystic neoplasms and two neuroendocrine tumors). All lesions were successfully ablated, and after a 6-month follow-up, two cysts had resolved, three cysts had a 50% reduction in size, and two neuroendocrine tumors developed central necrosis. No serious complications were observed. Further large-scale studies are warranted to assess the safety (particularly, rates of pancreatitis) and efficacy of this novel treatment modality. An 18-gauge RFA needle and a VIVA RF generator with a working length of 150 cm and a 10-mm electrode in the needle tip has also been studied in six patients with unresectable pancreatic cancer [16]. The procedure was technically successful in all patients with the only complication being post-procedure pain in two patients.
In recent years, multiple studies have demonstrated the feasibility and safety of fiducial marker placement under EUS guidance (with or without fluoroscopic assistance) to facilitate radiation therapy in pancreatic cancer [17,18]. Disadvantages of the typical back-loading technique used include being time-consuming to individually load small markers into the tip of a 19- or 22-gauge EUS needle by hand and variable deployment via the passage of stylet or saline through the needle. Typically 3 to 4 markers are needed; therefore, the needle has to be removed and reloaded for each marker. Recently, new EUS needles have been developed that have multiple fiducial markers already preloaded to save time and allow more accurate deployment with tactile feedback. Currently, the only Food and Drug Administration-approved fiducial needle is the Echotip fiducial needle manufactured by Cook Medical (Bloomington, IN, USA). This is a 22-gauge needle that contains 4 gold fiducial markers. Another preloaded fiducial needle model is currently being developed by Medtronic (Sunnyvale, CA, USA) in both 19- and 22-gauge needles and comes preloaded with 2 fiducial markers.
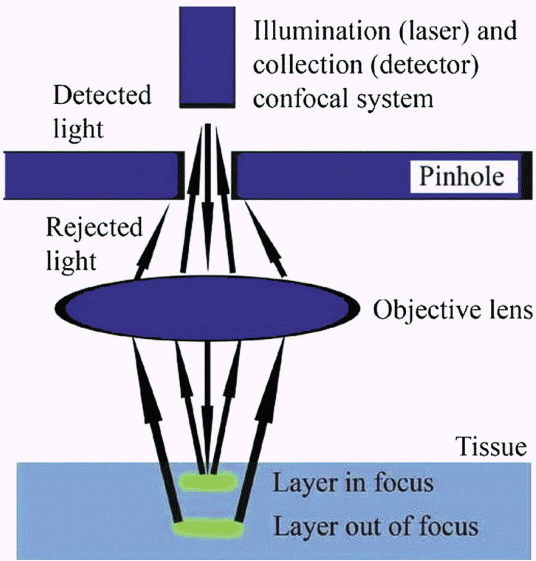
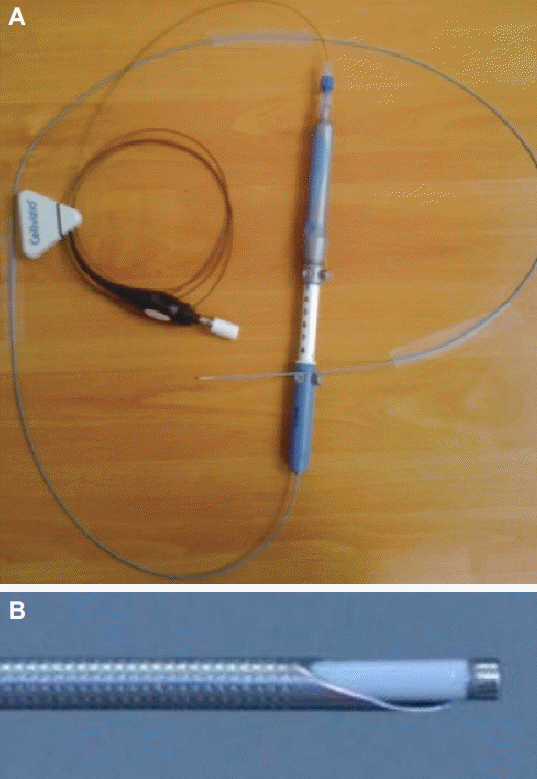
Confocal laser endomicroscopy (CLE) is an endoscopic technique developed to obtain high-resolution magnified images. The American Society for Gastrointestinal Endoscopy recently published a review of this innovative technology [19]. CLE is based on tissue illumination with a low-power laser with subsequent detection of the fluorescence of light reflected from the tissue through a pinhole (Fig. 3). The term confocal refers to the fact that both illumination and detection systems are in the same focal plane. Light is focused at a selected depth in the tissue of interest, and the reflected light is then refocused onto the detection system by the same lens. Only returning light refocused through the pinhole is detected, and light scattered at other angles is excluded from detection. This enables CLE to provide real-time cellular imaging and evaluation of tissue architecture during endoscopy through the use of topical and/or intravenous fluorescence contrast agents to generate images with a resolution similar to that of traditional histological examination. The probe-based CLE system is comprised of a low-powered laser with an integrated distal lens. The probe-based system (Cellvizio confocal probes; Mauna Kea Technologies, Paris, France) can create cross-sectional images at different depths, and the probe’s individual optical fibers function as pinholes. Needle-based confocal endomicroscopy (nCLE) utilizes the AQ-Flex 19 probe through a 19-gauge EUS needle (Fig. 4). The depth of imaging is 40 to 70 mm; the maximal field of view, 325 mm; and resolution, 3.5 mm.
Our center reported results from the initial feasibility trial studying the nCLE probe in pancreatic lesions in 2011 [20]. We demonstrated technical succes in 17/18 patients, had two cases of pancreatitis, established imaging criteria, and created a standardized protocol for use.
Subsequently, these results were validated in a prospective, multicenter study that included 65 patients [21]. The major finding of this study was that identification of papillary (finger-like) projections had 100% specificity for intraductal papillary mucinous neoplasms (IPMN). However, the study also showed that there were limitations due to variations in the location of the probe within a cyst and the epithelium of IPMNs themselves, which lead to a low sensitivity of 59%.
In 2015, Napoléon et al. [22] identified a new imaging criterion for diagnosing serous cystadenomas: superficial vascular network (SVN). Although nCLE again had a low sensitivity of 69%, if the SVN was visualized, it was 100% specific for serous cystadenomas, with a 100% positive predictive value.
The main limitation of the standard curvilinear array EUS (CLA-EUS) scope is that any device that exits the accessory channel is oblique to the endoscope axis, making the passage of accessories difficult. The standard CLA-EUS scope has an oblique view of 100° and an endoscopic view that is 55° rotated with respect to the axis of the endoscope and the ultrasound probe. Recently, a forward-viewing linear echoendoscope (FV-EUS; Olympus America, Center Valley, PA, USA) has been developed that shifts the orientation of both the endoscopic and ultrasound views from oblique to forward.
Earlier this year, Fuccio et al. [23] published an article illustrating the device specifications of the FV-EUS scope as described below. The FV-EUS ultrasound transducer is located adjacent to the working channel, at the tip of the endoscope, to display a forward-viewing image. This change now permits devices to come out parallel to the longitudinal axis of the endoscope, which could have the potential to allow for easier passage of devices and stents compared to standard CLA-EUS, by allowing for better visualization and increased application of mechanical force. However, the ultrasound scanning area is now limited to only 90° (compared to 180° in standard CLA-EUS scopes), and the 3.7 mm working channel lacks an elevator, which may represent a design limitation.
The FV-EUS scope has two potential advantages over standard CLA-EUS scopes: (1) elimination of the need to change endoscopes when a forward-viewing endoscope is necessary for endoscopic visualization, and (2) ability to reach or inspect areas of the GI lumen not easily accessible with the CLA-EUS. However, data to date has not shown a significant clinical benefit. One multicenter, prospective European study included 58 patients with PFCs who were randomized to drainage with either standard CLA-EUS or FV-EUS; however, their results did not show a difference in ease of drainage, safety, or efficacy between the two scope types [24].
In our own center, we used the FV-EUS to pass deeply into the afferent limb in a patient who underwent Whipple resection and then developed a post-operative abscess in the surgical bed. The abscess was adjacent to the afferent limb, approximately 60 cm distal to the gastrojejunal anastomosis. The added benefit of the enhanced forward-viewing endoscopic ability allowed us to pass the scope down the afferent limb and identify the collection. However, due to lack of an elevator, we could not obtain a satisfactory angle for puncture; therefore, we switched back to a standard CLA-EUS for guidewire puncture and stent placement [25].
Recent technological advances have made EUS a safe and effective method of providing a broad range of treatments and therapies previously not thought possible. The development of new stents for various procedures has expanded the ability to effectively collect fluids from the pancreas and gall bladder, perform gastrojejunostomy, and facilitate ERCP in gastric bypass patients. Additional tools allow novel therapies for the treatment of pancreatic tumors, such as RFA and placing fiducial markers to guide radiation therapy. Furthermore, advanced imaging modalities, such as nCLE, have allowed us to visualize the inside of pancreatic cystic lesions to assist with making a diagnosis. Finally, the development of new echoendoscopes, such as the FV-EUS, has the potential to allow better visualization of the GI tract and have added efficiency for therapeutic maneuvers. Through these additions to the endosonographer’s arsenal of tools, EUS continues its transition from a diagnostic modality to a more therapeutic procedure. As innovation in the field continues, we hope to see additional tools expanding the use of EUS well beyond its current limits with the ultimate goal of improving patient outcomes.
REFERENCES
1. Weilert F, Binmoeller KF. Specially designed stents for translumenal drainage. Gastrointest Interv. 2015; 4:40–45.

2. Binmoeller KF, Shah J. A novel lumen-apposing stent for transluminal drainage of nonadherent extraintestinal fluid collections. Endoscopy. 2011; 43:337–342.

3. Moon JH, Choi HJ, Kim DC, et al. A newly designed fully covered metal stent for lumen apposition in EUS-guided drainage and access: a feasibility study (with videos). Gastrointest Endosc. 2014; 79:990–995.

4. Wrobel PS, Kaplan J, Siddiqui AA. A new lumen-apposing metal stent for endoscopic transluminal drainage of peripancreatic fluid collections. Endosc Ultrasound. 2014; 3:203–204.

5. Itoi T, Binmoeller KF, Shah J, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc. 2012; 75:870–876.

6. Gornals JB, De la Serna-Higuera C, Sánchez-Yague A, Loras C, Sánchez-Cantos AM, Pérez-Miranda M. Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. Surg Endosc. 2013; 27:1428–1434.

7. Shah RJ, Shah JN, Waxman I, et al. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin Gastroenterol Hepatol. 2015; 13:747–752.

8. Irani S, Baron TH, Grimm IS, Khashab MA. EUS-guided gallbladder drainage with a lumen-apposing metal stent (with video). Gastrointest Endosc. 2015; 82:1110–1115.

9. Walter D, Teoh AY, Itoi T, et al. EUS-guided gall bladder drainage with a lumen-apposing metal stent: a prospective long-term evaluation. Gut. 2016; 65:6–8.

10. Teoh AY, Binmoeller KF, Lau JY. Single-step EUS-guided puncture and delivery of a lumen-apposing stent for gallbladder drainage using a novel cautery-tipped stent delivery system. Gastrointest Endosc. 2014; 80:1171.

11. Itoi T, Itokawa F, Uraoka T, et al. Novel EUS-guided gastrojejunostomy technique using a new double-balloon enteric tube and lumen-apposing metal stent (with videos). Gastrointest Endosc. 2013; 78:934–939.

12. Itoi T, Ishii K, Tanaka R, Umeda J, Tonozuka R. Current status and perspective of endoscopic ultrasonography-guided gastrojejunostomy: endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy (with videos). J Hepatobiliary Pancreat Sci. 2015; 22:3–11.

13. Itoi T, Ishii K, Ikeuchi N, et al. Prospective evaluation of endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy bypass (EPASS) for malignant gastric outlet obstruction. Gut. 2016; 65:193–195.

14. Kedia P, Tyberg A, Kumta NA, et al. EUS-directed transgastric ERCP for Roux-en-Y gastric bypass anatomy: a minimally invasive approach. Gastrointest Endosc. 2015; 82:560–565.

15. Pai M, Habib N, Senturk H, et al. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015; 7:52–59.

16. Song TJ, Seo DW, Lakhtakia S, et al. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest Endosc. 2015; Sep. 4. [Epub]. http://dx.doi.org/10.1016/j.gie.2015.08.048.

17. Choi JH, Seo DW, Park do H, Lee SK, Kim MH. Fiducial placement for stereotactic body radiation therapy under only endoscopic ultrasonography guidance in pancreatic and hepatic malignancy: practical feasibility and safety. Gut Liver. 2014; 8:88–93.

18. Fuccio L, Lami G, Guido A, Fabbri C. EUS-guided gold fiducial placement and migration rate. Gastrointest Endosc. 2014; 80:533–534.

19. ASGE Technology Committee. Confocal laser endomicroscopy. Gastrointest Endosc. 2014; 80:928–938.
20. Konda VJ, Aslanian HR, Wallace MB, Siddiqui UD, Hart J, Waxman I. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos). Gastrointest Endosc. 2011; 74:1049–1060.

21. Konda VJ, Meining A, Jamil LH, et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013; 45:1006–1013.

22. Napoléon B, Lemaistre AI, Pujol B, et al. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2015; 47:26–32.

23. Fuccio L, Attili F, Larghi A. Forward-viewing linear echoendoscope: a new option in the endoscopic ultrasound armamentarium (with video). J Hepatobiliary Pancreat Sci. 2015; 22:27–34.

Fig. 3.
Depiction of how confocal laser endomicroscopy images are obtained. Adapted from ASGE Technology Committee, with permission from Elsevier. [19].




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download