Abstract
Obscure gastrointestinal bleeding (OGIB) accounts for 5% of all gastrointestinal (GI) bleeding cases and is often caused by small bowel lesions. Capsule endoscopy (CE), which allows non-invasive visualization of the small bowel mucosa, has revolutionized the evaluation of OGIB. CE is preferred by both patients and physicians mainly because of its non-invasiveness, and is widely used as the first-line diagnostic modality for OGIB. The diagnostic yield of CE in OGIB has been reported to be in the range of 32% to 83%. Although no direct comparison has been made, a meta-analysis showed similar diagnostic yields between CE and double-balloon enteroscopy (DBE) for OGIB. However, CE could enhance the yield of subsequent DBE and serve as a guide for optimizing the insertion route for DBE. Even after negative CE, selected patients could benefit from second-look CE for OGIB. In terms of outcomes, a favorable clinical impact after CE has been reported in several studies. However, observations indicate that CE might not influence clinical outcomes directly, but rather play a role in selecting patients with OGIB who are likely to benefit from subsequent evaluation and intervention.
Obscure gastrointestinal bleeding (OGIB) is defined as bleeding of unknown origin that persists or recurs after negative evaluations including upper and lower endoscopies [1]. OGIB accounts for 5% of all cases of gastrointestinal (GI) bleeding, and is often caused by small bowel lesions [2-4]. However, the small bowel is now within the reach of GI endoscopy, owing to technological advances in endoscopy. As a result, new small bowel endoscopies, including capsule endoscopy (CE), double-balloon enteroscopy (DBE), and single-balloon enteroscopy, now play a crucial role in the evaluation and management of OGIB. Among these new technologies, CE was introduced first [5] and is preferred by both patients and physicians, mainly because of its non-invasiveness. CE also has a significantly higher diagnostic yield in patients with OGIB than do alternative diagnostic radiological or endoscopic modalities, including small bowel follow-through and push enteroscopy (not including balloon-assisted enteroscopy) [6-9]. Thus, CE is widely used as the first-line diagnostic modality for OGIB [2,10-13]. This article reviews the diagnostic role and long-term clinical outcomes of CE in patients with OGIB.
The diagnostic yield of CE in OGIB has been reported to be in the range of 32% to 83%, depending on the definition of positive findings and the type of bleeding investigated [4,14-22]. In a systemic review by Liao et al. [23], which involved 22,840 CE cases from 227 studies, OGIB was the most common indication (66.0%), and the pooled detection rate for OGIB was 60.5%. Recent studies have also reported positive CE results for OGIB in 51.5% to 70.2% of cases [24-26]. Although there are no prospective, randomized, controlled trials comparing CE and DBE, a meta-analysis showed similar diagnostic yields of 61.7% and 55.5% for CE and DBE (p=0.16) for OGIB [27]. Given that CE is usually performed prior to DBE, the results of this meta-analysis need to be interpreted in the context of its limitation.
Numerous factors associated with positive CE results have been identified. Patients with ongoing overt bleeding are more likely to yield positive CE results [28,29]. In terms of the mode of bleeding at presentation, the frequency of positive CE results is higher in patients with obscure-overt GI bleeding than in those with obscure-occult GI bleeding [22]. However, other studies have failed to identify an association between the mode of presentation and diagnostic yield [30-32]. In addition, the diagnostic yield is increased when CE is performed within 1 or 2 weeks from the previous bleeding episode [33,34]. Severe anemia and increased transfusion requirements also contribute to increased diagnostic yields in patients with OGIB, including chronic bleeding [34-37]. Finally, increasing age, anti-coagulation therapy, and liver comorbidities have also been identified as significant predictors of positive CE results [32]. Thus, careful patient selection might improve the diagnostic yield of CE in patients with OGIB.
Although the diagnostic yield of CE and DBE is similar, CE is usually performed prior to DBE for evaluating OGIB. Positive CE results increased the diagnostic yield of subsequent DBE in a meta-analysis involving 10 studies [27]. The diagnostic yield of subsequent DBE after positive CE results was 75.0% (95% confidence interval [CI], 60.1 to 90.0), while that after negative CE results was only 27.5% (95% CI, 16.7 to 37.8). In a prospective study on 60 consecutive patients undergoing DBE only after obtaining positive CE results for OGIB, the diagnostic yield was 75% [38]. These observations indicate that CE may be capable of selecting patients who are likely to benefit from DBE. In addition, CE may be capable of selecting the optimal insertion route for DBE. In a prospective study by Gay et al. [39], subsequent push-and-pull enteroscopy was performed after CE in patients with suspected small bowel diseases via the anal route when the capsule transit time from ingestion to arrival at the lesion was ≥75% of the total time from ingestion to arrival at the cecum. The positive and negative predictive values of this method for selecting the correct route for enteroscopy were 94.7% and 96.7%, respectively. Using this concept, Li et al. [40] evaluated the utility of CE for predicting the correct insertion route for DBE in the evaluation and treatment of small bowel lesions. They adopted the anal approach for DBE when the capsule transit time from the pylorus to the lesion was >60% of the total transit time from the pylorus to the ileocecal valve, and the accuracy of selecting the correct insertion route was 100%.
Given the low rebleeding rate after negative CE, some patients may be able to avoid further invasive evaluations such as balloon-assisted enteroscopy, unless they have ongoing bleeding or the clinical suspicion is significantly high. In addition, patients with negative CE results may benefit from repeated “second-look” CE. In a retrospective study on 82 patients who underwent second-look CE for several reasons, including recurrent GI, anemia, screening for polyposis syndromes, abdominal pain, and failure of initial CE [41], positive findings were found in 55% (n=45), resulting in a change in management in 71% of the patients (n=32). In a study by Viazis et al. [42], second-look CE was performed in patients with OGIB and negative CE results when a new overt bleeding episode or a drop in hemoglobin level ≥2 g/dL was reported. According to the logistic regression analysis, change from occult to overt bleeding and a drop in hemoglobin ≥4 g/dL were significant predictive factors of a diagnostic test. Thus, selected patients with severe recurrent bleeding or change from occult to overt bleeding after negative CE for OGIB should potentially undergo second-look CE prior to other small bowel investigations such as balloon-assisted enteroscopy.
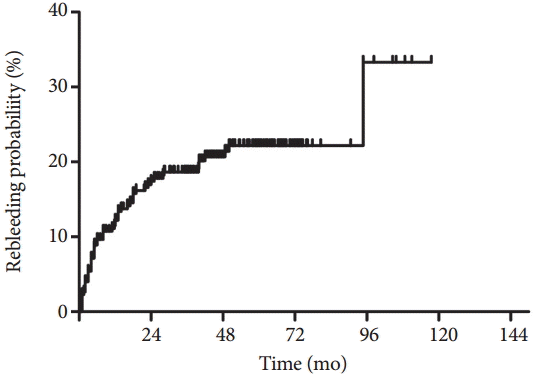
Several studies have reported a favorable clinical impact of CE on patients with OGIB. In a study by Pennazio et al. [28], CE results led to treatments that resolved bleeding in 86.9% of patients undergoing the procedure while experiencing active bleeding. In a small study involving 35 patients with OGIB, treatments were performed after CE in 37% of the patients [43]. However, another large study on 100 patients reported that specific interventions were made after CE in 75.8% of the patients with OGIB, and the clinical outcome was considered positive in 71.6% at the end of a mean follow-up period of 11.4 months [37]. Similarly, Katsinelos et al. [44] have reported that CE led to a specific therapy that resolved the underlying disease or improved the clinical condition in 71.4% of the patients during a mean follow-up duration of 11.8 months, and Hindryckx et al. [45] have reported that outcomes after CE were favorable in 66.3% of patients during a mean follow-up period of 635.5 days. In addition, CE changes the clinical management in 61.4% of patients with OGIB [31] and reduces hospitalization, additional tests/procedures, and units of blood transfused [22]. However, it is worth noting that the abovementioned clinical impacts of CE do not guarantee favorable long-term outcomes. In a study by Hindryckx et al. [45], clinical outcomes did not differ after CE, although favorable outcomes were reported overall in the study patients after CE. Furthermore, rebleeding occurred constantly after CE in patients with OGIB, according to a recent large study (Fig. 1) [25]. Thus, what is more important is the rebleeding rate revealed in long-term follow-up.
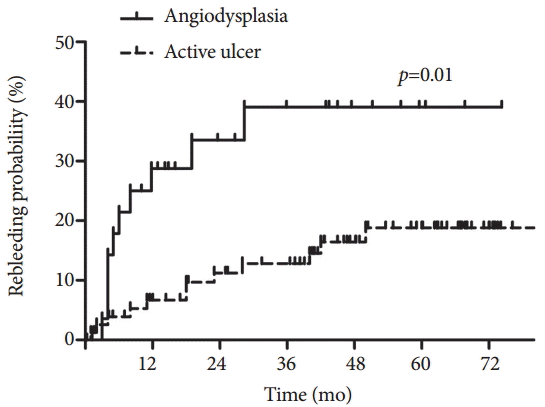
Over the past decade, several studies have reported long-term clinical outcomes of patients with OGIB. In most previous studies, patients with OGIB and negative CE results have had significantly lower rebleeding rates, ranging from 2.6% to 16.4% of those with positive CE results [19,24-26,42,46-49]. Although a significant risk of rebleeding seems to remain even in patients with negative CE results, patients with positive CE results bled significantly more than did those with negative CE results during a median follow-up duration of 24 months in a retrospective Italian single center study involving 696 CE cases (45.1% vs. 16.4%, p=0.001) [26]. However, in three Korean studies, there were no significant differences in rebleeding rates according to CE results. In a single center study involving 113 patients with OGIB, there was no significant difference in the cumulative rebleeding rate between patients with positive and negative CE results (36.8% vs. 22.8%, p=0.205) during a median follow-up period of 23.7 months [50]. In another Korean study with a median follow-up period of 31.7 months, the rebleeding rate did not differ between patients with positive and negative CE results (34.8% vs. 35.7%, p=0.989) [46]. A large nationwide study also showed similar rebleeding rates regardless of CE results during a mean follow-up period of 38.7 months [25]. These different long-term outcomes of Korean studies could be explained by the different positive CE findings from those of previous reports in Western countries [22,48]. In Korean studies, ulcer or erosion is the most common finding on CE, while angiodysplasia is the most common in Western studies. In a meta-analysis of DBE studies involving 5,268 suspected mid-GI bleeding cases, inflammatory lesions (37.6%) and vascular lesions (65.9%) were the most common findings in Eastern and Western countries, respectively [51]. Angiodysplasia has a multifocal character and is associated with a high risk of rebleeding [52-54], although it can be effectively treated with argon plasma coagulation [42,55,56]. According to a recent large study [25], angiodysplasia showed a higher rebleeding rate than did active ulcer (p=0.01), whereas active ulcer did not show a significantly different rebleeding rate than did negative findings (Fig. 2). Thus, the authors concluded that rebleeding after CE in patients with OGIB is determined by the specific cause rather than positive CE results or the application of interventional treatment. However, subgroup analysis of a Korean study showed that specific treatments after CE significantly reduced the rebleeding rate (hazard ratio, 0.111; 95% CI, 0.013 to 0.980; p=0.043) [46]. In another study, among patients with positive CE results, the patients who underwent therapeutic interventions showed significantly lower rebleeding rates than did those who did not undergo therapeutic interventions (9.5% vs. 40.0%, p=0.046) [57]. These observations indicate that CE does not directly influence long-term clinical outcomes, but rather plays a role in selecting patients who are likely to benefit from interventions.
As the diagnostic yield of CE and DBE is similar, CE is recommended prior to DBE in evaluating OGIB because of its non-invasiveness. Furthermore, CE results can enhance the yield of subsequent DBE and serve as a guide for selecting the insertion route for DBE. Although CE does not improve long-term clinical outcomes directly, it plays an important role in selecting patients who are likely to benefit from subsequent evaluation and intervention. In addition, CE can identify patients at a low rebleeding risk who may benefit from conservative management. Thus, CE appears to play an essential role in the management of patients with OGIB.
REFERENCES
1. Raju GS, Gerson L, Das A, Lewis B; American Gastroenterological Association. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007; 133:1697–1717.

2. Pennazio M, Eisen G, Goldfarb N; ICCE. ICCE consensus for obscure gastrointestinal bleeding. Endoscopy. 2005; 37:1046–1050.

3. Lin S, Rockey DC. Obscure gastrointestinal bleeding. Gastroenterol Clin North Am. 2005; 34:679–698.

4. Tang SJ, Haber GB. Capsule endoscopy in obscure gastrointestinal bleeding. Gastrointest Endosc Clin N Am. 2004; 14:87–100.

6. Ell C, Remke S, May A, Helou L, Henrich R, Mayer G. The first prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy. 2002; 34:685–689.

7. Voderholzer WA, Ortner M, Rogalla P, Beinhölzl J, Lochs H. Diagnostic yield of wireless capsule enteroscopy in comparison with computed tomography enteroclysis. Endoscopy. 2003; 35:1009–1014.

8. Costamagna G, Shah SK, Riccioni ME, et al. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002; 123:999–1005.

9. Hara AK, Leighton JA, Sharma VK, Fleischer DE. Small bowel: preliminary comparison of capsule endoscopy with barium study and CT. Radiology. 2004; 230:260–265.

10. Triester SL, Leighton JA, Leontiadis GI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005; 100:2407–2418.

11. Leighton JA, Triester SL, Sharma VK. Capsule endoscopy: a meta-analysis for use with obscure gastrointestinal bleeding and Crohn’s disease. Gastrointest Endosc Clin N Am. 2006; 16:229–250.

12. Shim KN, Moon JS, Chang DK, et al. Guideline for capsule endoscopy: obscure gastrointestinal bleeding. Clin Endosc. 2013; 46:45–53.

13. ASGE Standards of Practice, Fisher L, Lee Krinsky M, et al. The role of endoscopy in the management of obscure GI bleeding. Gastrointest Endosc. 2010; 72:471–479.

14. Concha R, Amaro R, Barkin JS. Obscure gastrointestinal bleeding: diagnostic and therapeutic approach. J Clin Gastroenterol. 2007; 41:242–251.
15. Mylonaki M, Fritscher-Ravens A, Swain P. Wireless capsule endoscopy: a comparison with push enteroscopy in patients with gastroscopy and colonoscopy negative gastrointestinal bleeding. Gut. 2003; 52:1122–1126.

16. Mata A, Bordas JM, Feu F, et al. Wireless capsule endoscopy in patients with obscure gastrointestinal bleeding: a comparative study with push enteroscopy. Aliment Pharmacol Ther. 2004; 20:189–194.

17. Rastogi A, Schoen RE, Slivka A. Diagnostic yield and clinical outcomes of capsule endoscopy. Gastrointest Endosc. 2004; 60:959–964.

18. Calabrese C, Liguori G, Gionchetti P, et al. Obscure gastrointestinal bleeding: single centre experience of capsule endoscopy. Intern Emerg Med. 2013; 8:681–687.

19. Iwamoto J, Mizokami Y, Shimokobe K, et al. The clinical outcome of capsule endoscopy in patients with obscure gastrointestinal bleeding. Hepatogastroenterology. 2011; 58:301–305.
20. Goenka MK, Majumder S, Kumar S, Sethy PK, Goenka U. Single center experience of capsule endoscopy in patients with obscure gastrointestinal bleeding. World J Gastroenterol. 2011; 17:774–778.

21. Zhang BL, Fang YH, Chen CX, Li YM, Xiang Z. Single-center experience of 309 consecutive patients with obscure gastrointestinal bleeding. World J Gastroenterol. 2009; 15:5740–5745.
22. Carey EJ, Leighton JA, Heigh RI, et al. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 2007; 102:89–95.

23. Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010; 71:280–286.

24. Katsinelos P, Lazaraki G, Gkagkalis A, et al. The role of capsule endoscopy in the evaluation and treatment of obscure-overt gastrointestinal bleeding during daily clinical practice: a prospective multicenter study. Scand J Gastroenterol. 2014; 49:862–870.

25. Min YW, Kim JS, Jeon SW, et al. Long-term outcome of capsule endoscopy in obscure gastrointestinal bleeding: a nationwide analysis. Endoscopy. 2014; 46:59–65.

26. Riccioni ME, Urgesi R, Cianci R, et al. Negative capsule endoscopy in patients with obscure gastrointestinal bleeding reliable: recurrence of bleeding on long-term follow-up. World J Gastroenterol. 2013; 19:4520–4525.

27. Teshima CW, Kuipers EJ, van Zanten SV, Mensink PB. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: an updated meta-analysis. J Gastroenterol Hepatol. 2011; 26:796–801.

28. Pennazio M, Santucci R, Rondonotti E, et al. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004; 126:643–653.

29. Ge ZZ, Chen HY, Gao YJ, Hu YB, Xiao SD. Best candidates for capsule endoscopy for obscure gastrointestinal bleeding. J Gastroenterol Hepatol. 2007; 22:2076–2080.

30. Selby W. Can clinical features predict the likelihood of finding abnormalities when using capsule endoscopy in patients with GI bleeding of obscure origin? Gastrointest Endosc. 2004; 59:782–787.

31. Redondo-Cerezo E, Pérez-Vigara G, Pérez-Sola A, et al. Diagnostic yield and impact of capsule endoscopy on management of patients with gastrointestinal bleeding of obscure origin. Dig Dis Sci. 2007; 52:1376–1381.

32. Sidhu R, Sanders DS, Kapur K, Leeds JS, McAlindon ME. Factors predicting the diagnostic yield and intervention in obscure gastrointestinal bleeding investigated using capsule endoscopy. J Gastrointestin Liver Dis. 2009; 18:273–278.
33. Bresci G, Parisi G, Bertoni M, Tumino E, Capria A. The role of video capsule endoscopy for evaluating obscure gastrointestinal bleeding: usefulness of early use. J Gastroenterol. 2005; 40:256–259.

34. Esaki M, Matsumoto T, Yada S, et al. Factors associated with the clinical impact of capsule endoscopy in patients with overt obscure gastrointestinal bleeding. Dig Dis Sci. 2010; 55:2294–2301.

35. May A, Wardak A, Nachbar L, Remke S, Ell C. Influence of patient selection on the outcome of capsule endoscopy in patients with chronic gastrointestinal bleeding. J Clin Gastroenterol. 2005; 39:684–688.

36. Parikh DA, Mittal M, Leung FW, Mann SK. Improved diagnostic yield with severity of bleeding. J Dig Dis. 2011; 12:357–363.

37. Estévez E, González-Conde B, Vázquez-Iglesias JL, et al. Diagnostic yield and clinical outcomes after capsule endoscopy in 100 consecutive patients with obscure gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2006; 18:881–888.
38. Kaffes AJ, Siah C, Koo JH. Clinical outcomes after double-balloon enteroscopy in patients with obscure GI bleeding and a positive capsule endoscopy. Gastrointest Endosc. 2007; 66:304–309.

39. Gay G, Delvaux M, Fassler I. Outcome of capsule endoscopy in determining indication and route for push-and-pull enteroscopy. Endoscopy. 2006; 38:49–58.

40. Li X, Chen H, Dai J, Gao Y, Ge Z. Predictive role of capsule endoscopy on the insertion route of double-balloon enteroscopy. Endoscopy. 2009; 41:762–766.

41. Svarta S, Segal B, Law J, et al. Diagnostic yield of repeat capsule endoscopy and the effect on subsequent patient management. Can J Gastroenterol. 2010; 24:441–444.

42. Viazis N, Papaxoinis K, Vlachogiannakos J, Efthymiou A, Theodoropoulos I, Karamanolis DG. Is there a role for second-look capsule endoscopy in patients with obscure GI bleeding after a nondiagnostic first test? Gastrointest Endosc. 2009; 69:850–856.

43. Ben Soussan E, Antonietti M, Hervé S, et al. Diagnostic yield and therapeutic implications of capsule endoscopy in obscure gastrointestinal bleeding. Gastroenterol Clin Biol. 2004; 28:1068–1073.

44. Katsinelos P, Chatzimavroudis G, Terzoudis S, et al. Diagnostic yield and clinical impact of capsule endoscopy in obscure gastrointestinal bleeding during routine clinical practice: a single-center experience. Med Princ Pract. 2011; 20:60–65.

45. Hindryckx P, Botelberge T, De Vos M, De Looze D. Clinical impact of capsule endoscopy on further strategy and long-term clinical outcome in patients with obscure bleeding. Gastrointest Endosc. 2008; 68:98–104.

46. Park JJ, Cheon JH, Kim HM, et al. Negative capsule endoscopy without subsequent enteroscopy does not predict lower long-term rebleeding rates in patients with obscure GI bleeding. Gastrointest Endosc. 2010; 71:990–997.

47. Tan W, Ge ZZ, Gao YJ, et al. Long-term outcome in patients with obscure gastrointestinal bleeding after capsule endoscopy. J Dig Dis. 2015; 16:125–134.

48. Lai LH, Wong GL, Chow DK, Lau JY, Sung JJ, Leung WK. Long-term follow-up of patients with obscure gastrointestinal bleeding after negative capsule endoscopy. Am J Gastroenterol. 2006; 101:1224–1228.

49. Macdonald J, Porter V, McNamara D. Negative capsule endoscopy in patients with obscure GI bleeding predicts low rebleeding rates. Gastrointest Endosc. 2008; 68:1122–1127.

50. Koh SJ, Im JP, Kim JW, et al. Long-term outcome in patients with obscure gastrointestinal bleeding after negative capsule endoscopy. World J Gastroenterol. 2013; 19:1632–1638.

51. Xin L, Liao Z, Jiang YP, Li ZS. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: a systematic review of data over the first decade of use. Gastrointest Endosc. 2011; 74:563–570.

52. Richter JM, Christensen MR, Colditz GA, Nishioka NS. Angiodysplasia. Natural history and efficacy of therapeutic interventions. Dig Dis Sci. 1989; 34:1542–1546.
53. Saurin JC, Delvaux M, Vahedi K, et al. Clinical impact of capsule endoscopy compared to push enteroscopy: 1-year follow-up study. Endoscopy. 2005; 37:318–323.

54. Magalhães-Costa P, Bispo M, Santos S, Couto G, Matos L, Chagas C. Re-bleeding events in patients with obscure gastrointestinal bleeding after negative capsule endoscopy. World J Gastrointest Endosc. 2015; 7:403–410.

55. Samaha E, Rahmi G, Landi B, et al. Long-term outcome of patients treated with double balloon enteroscopy for small bowel vascular lesions. Am J Gastroenterol. 2012; 107:240–246.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download