Abstract
Patients with inflammatory bowel disease have significantly increased in recent decades in Korea. Intestinal tuberculosis (ITB) and intestinal Behcet’s disease (BD), which should be differentiated from Crohn’s disease (CD), are more frequent in Korea than in the West. Thus, the accurate diagnosis of these inflammatory diseases is problematic in Korea and clinicians should fully understand their clinical and endoscopic characteristics. Ulcerative colitis mostly presents with rectal inflammation and continuous lesions, while CD presents with discontinuous inflammatory lesions and frequently involves the ileocecal area. Involvement of fewer than four segments, a patulous ileocecal valve, transverse ulcers, and scars or pseudopolyps are more frequently seen in ITB than in CD. A few ulcers with discrete margins are a typical endoscopic finding of intestinal BD. However, the differential diagnosis is difficult in many clinical situations because typical endoscopic findings are not always observed. Therefore, clinicians should also consider symptoms and laboratory, pathological, and radiological findings, in addition to endoscopic findings.
Go to : 
Inflammatory bowel disease (IBD) is a chronic and idiopathic inflammatory disease of the digestive tract showing a remitting and relapsing disease course. Ulcerative colitis (UC) and Crohn’s disease (CD) are two major forms of IBD. The number of patients with IBD has rapidly increased in recent years and is increasingly prominent in Korea [1]. However, many clinicians do not yet fully understand the clinical and endoscopic characteristics of IBD so that diagnosis is frequently delayed or incorrect in many cases. Accurate diagnosis of IBD is an important problem in Korea due to the higher prevalence of intestinal tuberculosis (ITB) and intestinal Behcet’s disease (BD) than in the West [2,3].
Guidelines for diagnosis of IBD were established by the IBD Study Group of the Korean Association for the Study of Intestinal Diseases in 2009 [3-6]. In this review, we delineate typical endoscopic findings and the differential diagnostic features of UC, CD, ITB, and intestinal BD based on these guidelines.
Go to : 
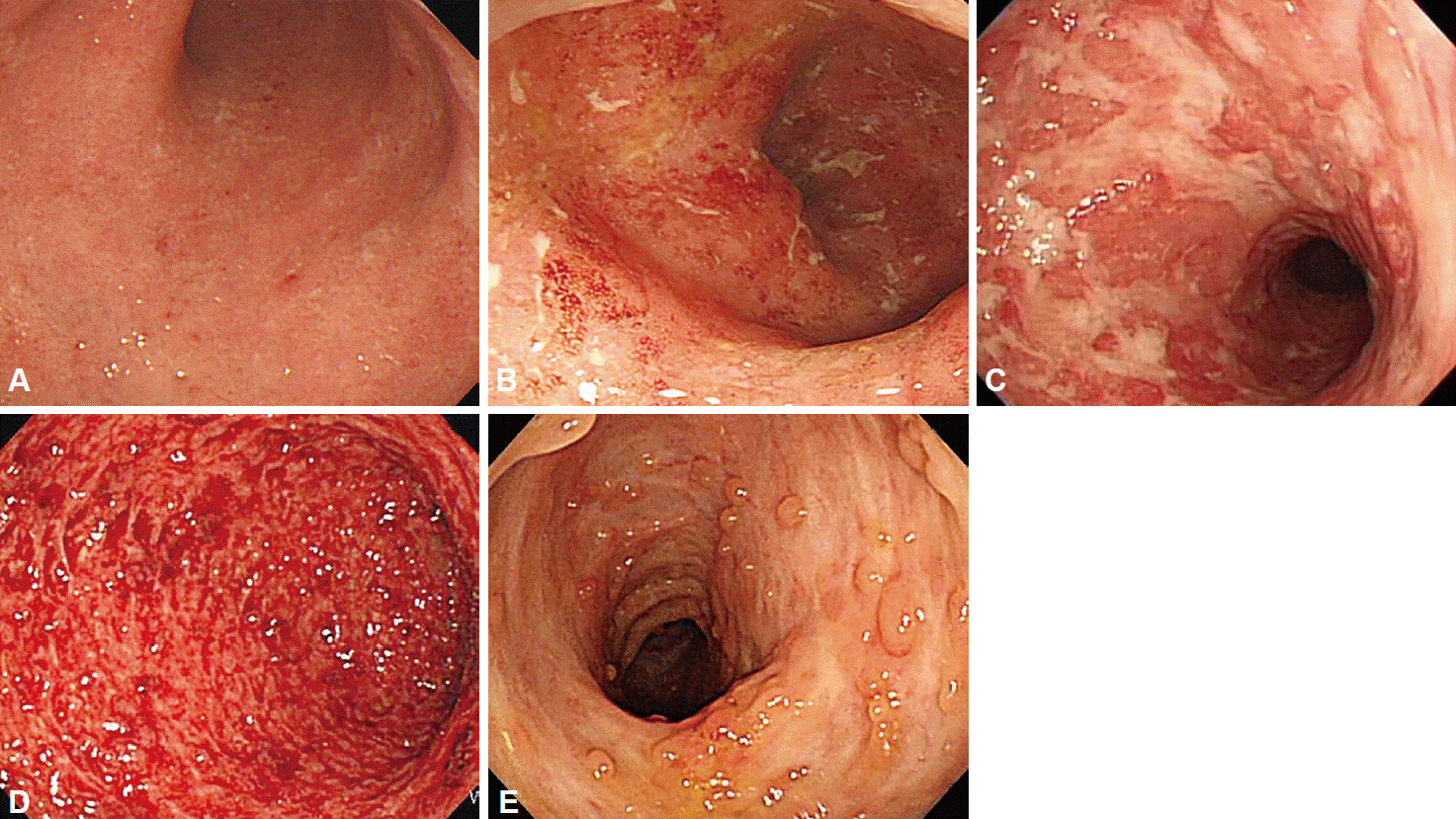
The inflammation involves the rectum in most cases and spreads proximally. The typical endoscopic findings in patients with UC include edematous mucosa, erythema, loss of vascular markings, and mucosal friability [4]. More severe cases may be associated with erosions, ulcers, and spontaneous bleeding. Luminal narrowing and pseudopolyps may occur due to chronic inflammation, which results in mucosal atrophy (Fig. 1) [7-9]. Focal inflammation around the appendiceal orifice is observed in up to 75% of patients with UC [10].
However, these typical findings are not always present. A Korean study revealed that atypical distribution of inflammation was seen in 20% of newly diagnosed UC patients during initial colonoscopy: 3.3% had segmental UC with rectal sparing and 15.8% presented with segmental skip lesions without appendiceal orifice inflammation [11]. Although inflammation in UC patients is mostly limited to the colon, ileal inflammation (backwash ileitis) may infrequently be observed [12]. In this case, physicians should consider small bowel evaluation to differentiate CD from UC [13].
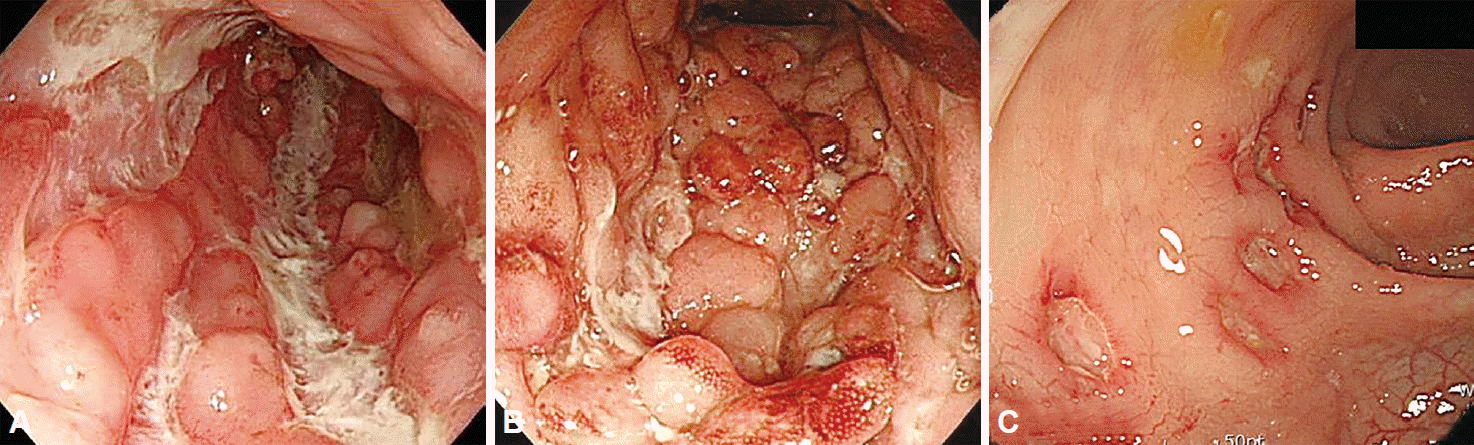
Typical endoscopic findings in CD include discontinuous distribution of longitudinal ulcers (defined as ≥4 to 5 cm ulcers in the Japanese criteria), cobblestone appearance, and/or small aphthous ulcerations arranged in a longitudinal fashion (Fig. 2) [5,14]. A Korean study reported longitudinal ulcers in 37.2%, cobblestone appearance in 23.9%, and aphthous ulcers in 59.3% of newly diagnosed CD cases. Non-caseating granuloma, a biopsy feature suggestive of CD, may be noted in only 13% to 36% of patients with CD so that sensitivity of biopsy is low [15].
Esophagogastroduodenoscopy is not routinely recommended in CD patients unless upper gastrointestinal symptoms are present [5]. Gastroduodenal CD presents with nonspecific lesions such as erosions, erythema, or ulcers [16]. A bamboo joint-like appearance can be seen on endoscopy, characterized by swollen, longitudinal folds traversed by erosive fissures or linear furrows on the lesser curvature of the gastric body and cardia [17].
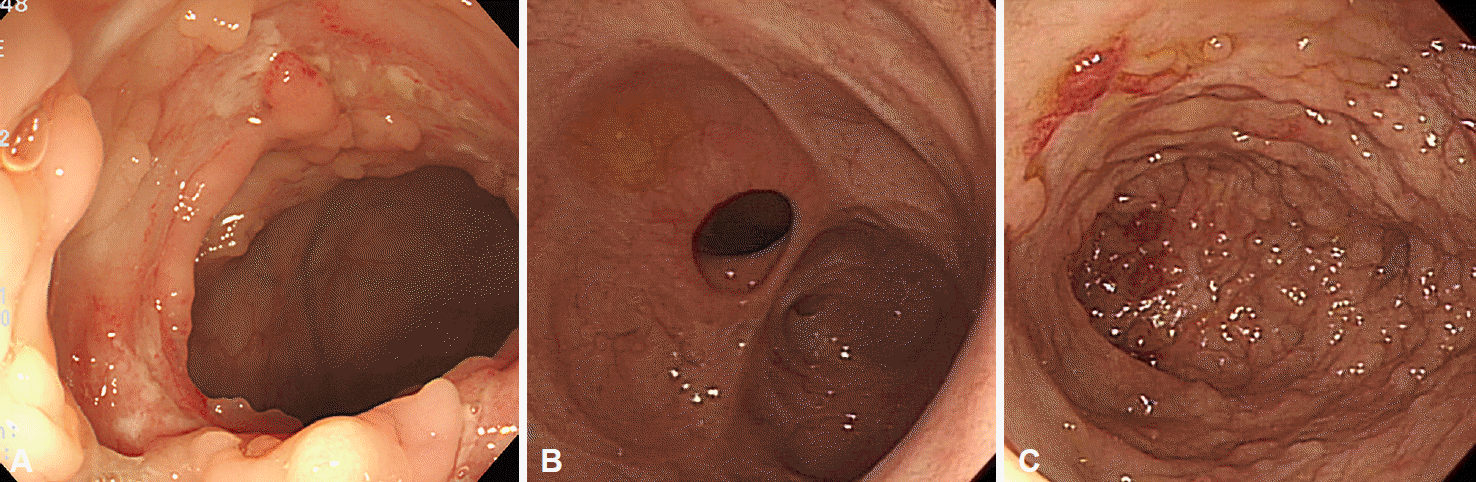
ITB frequently involves the ileocecal area and ascending colon. Endoscopic findings of ITB may include transverse ulcers, nodules with inflammatory changes of surrounding mucosa, pseudopolyps, and/or distorted and strictured ileocecal valves (Fig. 3) [3,18,19]. Although the typical histologic feature of caseating granuloma, positive acid-fast staining, and/or isolation of Mycobacterium tuberculosis from the culture of biopsy specimens can confirm ITB, these findings are present in fewer than 50% of patients [20,21]. Therefore, acquisition of more than three biopsy samples from the margin and base of ulcers is recommended to increase the diagnostic yield [3].
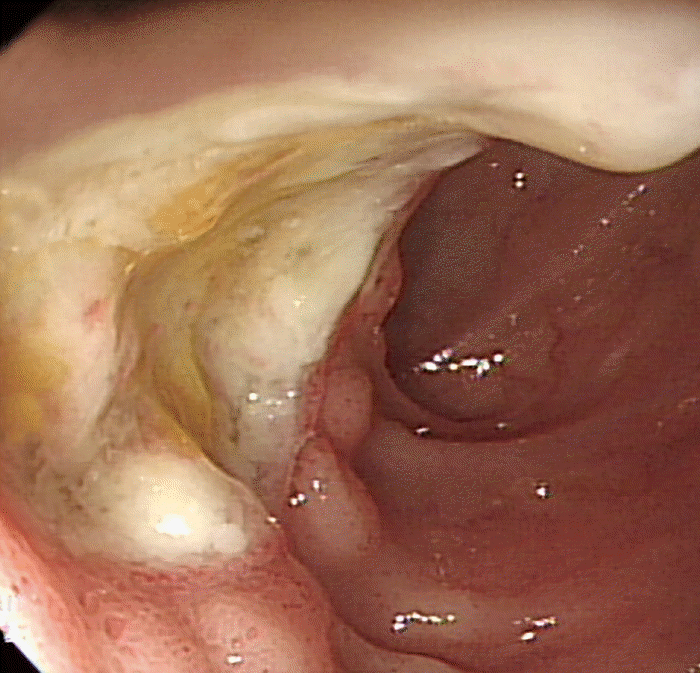
Typical endoscopic findings in intestinal BD are a few, large, round, deep, and discrete ulcers with elevated margins in the ileocecal area (Fig. 4). Other atypical findings such as aphthous ulcers, irregular/geographic-shaped ulcers with focal distribution, and diffuse ulceration can be present in patients with intestinal BD [6,22].
Go to : 
The differential diagnosis of UC includes infectious colitis, ischemic colitis, colitis due to drugs, radiation colitis, and solitary rectal ulcer syndrome [4,23]. A history of preceding abdominal pain, medication use, radiation therapy, or straining during defecation can be helpful [4].
Clinicians should always keep in mind that there is no specific endoscopic finding of UC. Infectious colitis can present with endoscopic features similar to those of UC. Thus, to make the correct diagnosis, onset and duration of symptoms should be considered together. Microbiological culture can also be helpful for differentiating UC from infectious colitis. Indeed, in a multinational web-based survey conducted by the Asian Organization for Crohn’s and Colitis, more than half of Asian gastroenterologists reported always or usually performing microbiological culture for suspected UC [24].
UC mostly presents with rectal inflammation and continuous lesions, while CD presents with discontinuous inflammatory lesions that frequently involve the ileocecal area. Shallow and indiscrete ulcers that involve only the mucosa, with edematous and erythematous changes in the surrounding area may suggest UC [22]. CD can involve not only the colon but also the small bowel, and frequently presents with deep ulcers. The deep ulcers with a longitudinal array create a cobblestone appearance. Sparing of the rectum, presence of perianal disease, and occurrence of strictures and fistulas suggest CD.
Use of serologic markers may help in the differential diagnosis of UC and CD [25,26]. Anti-Saccharomyces cerevisiae antibodies (ASCA) can be detected in 35% to 50% of CD patients, but in only 1% of patients with UC [26]. On the other hand, perinuclear antineutrophil cytoplasmic antibodies (pANCA) are detected more frequently in patients with UC. The sensitivity of pANCA+ for UC was reportedly 55.3%, and ASCA+ in combination with pANCA– resulted in 54.6% sensitivity for detection of CD [25]. Considering the relatively low sensitivity, serologic test can be the adjunctive tool when differentiation between CD and UC is clinically difficult (Table 1).
Differential Diagnosis of UC and CD
The presence of longitudinal ulcers is a typical endoscopic finding in CD, whereas transverse ulcers are common in ITB (Table 2). However, since these typical findings are not always present, it is often difficult to differentiate between the two diseases [23]. In a Korean study that assessed the diagnostic value of various colonoscopic findings in CD and ITB, anorectal lesions, longitudinal ulcers, aphthous ulcers, and cobblestone appearance were seen frequently in CD, whereas involvement of fewer than four segments, a patulous ileocecal valve, transverse ulcers, and scars or pseudopolyps were observed commonly in ITB [27]. Using these parameters, the correct diagnosis was made in 87.5% of patients, with a positive predictive value of 94.9% for CD and 88.9% for ITB, respectively. However, this analysis method is still not validated, and further studies are needed.
Endoscopic Characteristics of CD and ITB
The typical histologic finding of caseating granuloma and positive acid-fast staining are seen in fewer than 30% of ITB patients [28], but chest X-ray and interferon-γ assay may aid in the diagnosis of ITB. In fact, 67% of ITB patients had active pulmonary TB in a Korean study [29]. Therefore, chest X-ray is an essential test in ITB. Interferon-γ assay may also be useful as a supplementary diagnostic tool for ITB. A recent Korean study reported that 66% of patients with ITB had a positive interferon-γ assay (QuantiFERON-TB Gold test), compared to 9.7% of patients with CD [30].
If accurate diagnosis of the two diseases remains unclear despite the above methods, performing follow-up colonoscopy after empirical anti-TB therapy can be helpful [3].
A Korean study suggested an algorithm for the differential diagnosis of CD and intestinal BD according to endoscopic features. Ulcer shape, distribution, number, margins, and border contours, and the presence of aphthous, cobblestone, perianal, and strictured lesions enabled correct diagnosis of intestinal BD or CD in 92% of a study population [31]. Among these parameters, the most sensitive finding indicative of intestinal BD was the absence of a cobblestone appearance, and the most specific was a round ulcer shape. In addition, distribution patterns in patients with intestinal BD are more focal than in patients with CD. Therefore, a round ulcer or focally distributed lesions in the intestinal tract can suggest intestinal BD.
Another Korean study proposed simple criteria for the diagnosis of intestinal BD using systemic and colonoscopic features. Five or fewer lesions, oval shape, deep penetration, discrete border, and ileocecal location were regarded as typical ulcerations in BD. Overall, the positive predictive value and diagnostic accuracy using these criteria were 86.1% and 91.1%, respectively [32].
Go to : 
Colonoscopy is the principal test for diagnosing IBD. Clinicians can accurately diagnose most cases by fully understanding the typical endoscopic findings. In some cases, however, it is difficult to differentiate IBD due to an atypical presentation. Therefore, not only endoscopic features but also clinical symptoms, as well as laboratory, pathological, and radiological findings should be considered.
Go to : 
REFERENCES
1. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549.

3. Kim YS, Kim YH, Lee KM, Kim JS, Park YS; IBD Study Group of the Korean Association of the Study of Intestinal Diseases. Diagnostic guideline of intestinal tuberculosis. Korean J Gastroenterol. 2009; 53:177–186.
4. Choi CH, Jung SA, Lee BI, et al. Diagnostic guideline of ulcerative colitis. Korean J Gastroenterol. 2009; 53:145–160.
5. Ye BD, Jang BI, Jeen YT, et al. Diagnostic guideline of Crohn’s disease. Korean J Gastroenterol. 2009; 53:161–176.
6. Cheon JH, Shin SJ, Kim SW, et al. Diagnosis of intestinal Behcet’s disease. Korean J Gastroenterol. 2009; 53:187–193.
7. Waye JD. The role of colonoscopy in the differential diagnosis of inflammatory bowel disease. Gastrointest Endosc. 1977; 23:150–154.

8. Waye JD. Endoscopy in inflammatory bowel disease: indications and differential diagnosis. Med Clin North Am. 1990; 74:51–65.

9. Jalan KN, Walker RJ, Sircus W, McManus JP, Prescott RJ, Card WI. Pseudopolyposis in ulcerative colitis. Lancet. 1969; 2:555–559.

10. D’Haens G, Geboes K, Peeters M, Baert F, Ectors N, Rutgeerts P. Patchy cecal inflammation associated with distal ulcerative colitis: a prospective endoscopic study. Am J Gastroenterol. 1997; 92:1275–1279.
11. Park SH, Yang SK, Park SK, et al. Atypical distribution of inflammation in newly diagnosed ulcerative colitis is not rare. Can J Gastroenterol Hepatol. 2014; 28:125–130.

12. Haskell H, Andrews CW Jr, Reddy SI, et al. Pathologic features and clinical significance of “backwash” ileitis in ulcerative colitis. Am J Surg Pathol. 2005; 29:1472–1481.

13. Stange EF, Travis SP, Vermeire S, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. Gut. 2006; 55 Suppl 1:i1–i15.

14. Yao T. New criteria for the diagnosis of Crohn’s disease. Stomach Intest. 1996; 31:451–464.
15. Park JB, Yang SK, Myung SJ, et al. Clinical characteristics at diagnosis and course of Korean patients with Crohn’s disease. Korean J Gastroenterol. 2004; 43:8–17.
16. Kang MS, Park DI, Park JH, et al. Bamboo joint-like appearance of stomach in Korean patients with Crohn’s disease. Korean J Gastroenterol. 2006; 48:395–400.
17. Yokota K, Saito Y, Einami K, et al. A bamboo joint-like appearance of the gastric body and cardia: possible association with Crohn’s disease. Gastrointest Endosc. 1997; 46:268–272.

18. Naga MI, Okasha HH, Ismail Z, El-Fatatry M, Hassan S, Monir BE. Endoscopic diagnosis of colonic tuberculosis. Gastrointest Endosc. 2001; 53:789–793.

19. Singh V, Kumar P, Kamal J, Prakash V, Vaiphei K, Singh K. Clinicocolonoscopic profile of colonic tuberculosis. Am J Gastroenterol. 1996; 91:565–568.
20. Hoshino M, Shibata M, Goto N, et al. A clinical study of tuberculous colitis. Gastroenterol Jpn. 1979; 14:299–305.

21. Lee YJ, Yang SK, Myung SJ, et al. The usefulness of colonoscopic biopsy in the diagnosis of intestinal tuberculosis and pattern of concomitant extra-intestinal tuberculosis. Korean J Gastroenterol. 2004; 44:153–159.
23. Lee HS, Choe J, Lee HJ, et al. Change in the diagnosis of inflammatory bowel disease: a hospital-based cohort study from Korea. Intest Res. 2016; 14:258–263.

24. Kim ES, Chen M, Lee J, Lee CK, Kim YS. Diagnosis of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2nd Asian Organization for Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:224–230.
25. Reese GE, Constantinides VA, Simillis C, et al. Diagnostic precision of anti-Saccharomyces cerevisiae antibodies and perinuclear antineutrophil cytoplasmic antibodies in inflammatory bowel disease. Am J Gastroenterol. 2006; 101:2410–2422.

26. Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007; 133:1670–1689.

27. Lee YJ, Yang SK, Byeon JS, et al. Analysis of colonoscopic findings in the differential diagnosis between intestinal tuberculosis and Crohn’s disease. Endoscopy. 2006; 38:592–597.

28. Alvares JF, Devarbhavi H, Makhija P, Rao S, Kottoor R. Clinical, colonoscopic, and histological profile of colonic tuberculosis in a tertiary hospital. Endoscopy. 2005; 37:351–356.

29. Kim KM, Lee A, Choi KY, Lee KY, Kwak JJ. Intestinal tuberculosis: clinicopathologic analysis and diagnosis by endoscopic biopsy. Am J Gastroenterol. 1998; 93:606–609.

30. Kim YS, Kim YH, Kim WH, et al. Diagnostic utility of anti-Saccharomyces cerevisiae antibody (ASCA) and interferon-γ assay in the differential diagnosis of Crohn’s disease and intestinal tuberculosis. Clin Chim Acta. 2011; 412:1527–1532.

31. Lee SK, Kim BK, Kim TI, Kim WH. Differential diagnosis of intestinal Behcet’s disease and Crohn’s disease by colonoscopic findings. Endoscopy. 2009; 41:9–16.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download