1. Kamath P, Bhojwani KM, Prasannaraj T, Abhijith K. Foreign bodies in the aerodigestive tract: a clinical study of cases in the coastal belt of South India. Am J Otolaryngol. 2006; 27:373–377.
2. Park JH, Park CH, Park JH, et al. Review of 209 cases of foreign bodies in the upper gastrointestinal tract and clinical factors for successful endoscopic removal. Korean J Gastroenterol. 2004; 43:226–233.
3. Moon JS, Kim YH, Song TJ, Ryu HS, Hyun JH. The foreign bodies in the upper gastrointestinal tract diagnosed by endoscopy. Korean J Gastrointest Endosc. 1990; 10:305–315.
4. Lee MS, Ra DJ, Kim JH, Cho SW, Shim CS. A review of the endoscopic extraction in 52 cases of the upper gastrointestinal foreign bodies. Korean J Gastrointest Endosc. 1990; 10:47–52.
5. Webb WA. Management of foreign bodies of the upper gastrointestinal tract: update. Gastrointest Endosc. 1995; 41:39–51.

6. Hachimi-Idrissi S, Corne L, Vandenplas Y. Management of ingested foreign bodies in childhood: our experience and review of the literature. Eur J Emerg Med. 1998; 5:319–323.
7. Park SM, Chung MS, Choi JY, et al. Gastrointestinal foreign bodies: review of 118 cases. Korean J Gastroenterol. 1999; 33:464–472.
8. Blaho KE, Merigian KS, Winbery SL, Park LJ, Cockrell M. Foreign body ingestions in the Emergency Department: case reports and review of treatment. J Emerg Med. 1998; 16:21–26.

9. Kamal I, Thompson J, Paquette DM. The hazards of vinyl glove ingestion in the mentally retarded patient with pica: new implications for surgical management. Can J Surg. 1999; 42:201–204.
10. Abdullah BJ, Teong LK, Mahadevan J, Jalaludin A. Dental prosthesis ingested and impacted in the esophagus and orolaryngopharynx. J Otolaryngol. 1998; 27:190–194.
11. Longstreth GF, Longstreth KJ, Yao JF. Esophageal food impaction: epidemiology and therapy. A retrospective, observational study. Gastrointest Endosc. 2001; 53:193–198.

12. Nandi P, Ong GB. Foreign body in the oesophagus: review of 2394 cases. Br J Surg. 1978; 65:5–9.

13. Ono J. Foreign bodies in air and food passages in the Japanese. Arch Otolaryngol. 1965; 81:416–420.

14. Lai AT, Chow TL, Lee DT, Kwok SP. Risk factors predicting the development of complications after foreign body ingestion. Br J Surg. 2003; 90:1531–1535.

15. Carp L. Foreign bodies in the intestine. Ann Surg. 1927; 85:575–591.

16. Dray X, Cattan P. Foreign bodies and caustic lesions. Best Pract Res Clin Gastroenterol. 2013; 27:679–689.

17. Pfau PR. Removal and management of esophageal foreign bodies. Tech Gastrointest Endosc. 2014; 16:32–39.

18. Birk M, Bauerfeind P, Deprez PH, et al. Removal of foreign bodies in the upper gastrointestinal tract in adults: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016; 48:489–496.

19. Palta R, Sahota A, Bemarki A, Salama P, Simpson N, Laine L. Foreign-body ingestion: characteristics and outcomes in a lower socioeconomic population with predominantly intentional ingestion. Gastrointest Endosc. 2009; 69(3 Pt 1):426–433.

20. Weiland ST, Schurr MJ. Conservative management of ingested foreign bodies. J Gastrointest Surg. 2002; 6:496–500.

21. Schwartz GF, Polsky HS. Ingested foreign bodies of the gastrointestinal tract. Am Surg. 1976; 42:236–238.
22. Zhang S, Cui Y, Gong X, Gu F, Chen M, Zhong B. Endoscopic management of foreign bodies in the upper gastrointestinal tract in South China: a retrospective study of 561 cases. Dig Dis Sci. 2010; 55:1305–1312.

23. Park SJ, Jeon SM, Shin HD, et al. Risk factors for severe complications in patients with esophageal foreign bodies. Korean J Med. 2015; 89:537–547.

24. Kim HJ, Lee OJ, Min HJ, et al. Endoscopic treatment of esophageal foreign bodies in adult: management of 257 cases. Korean J Gastrointest Endosc. 2004; 29:51–57.
25. Park YK, Kim KO, Yang JH, Lee SH, Jang BI. Factors associated with development of complications after endoscopic foreign body removal. Saudi J Gastroenterol. 2013; 19:230–234.

26. Kim HU, Song HJ. Clinical characteristics of an esophageal fish bone foreign body from Chromis notata. J Korean Med Sci. 2012; 27:1208–1214.
27. Kim HU, Song HJ, Choi EK, Cho YK, Song BC. A case of a pharyngeal impacted fish bone foreign body detected by finger palpation. Korean J Gastrointest Endosc. 2011; 42:228–231.
28. Lee KS, Lee SH, Suh IS, Kim EH, Choi YW, Lee YU. Review of 78 cases foreign body in the upper gastrointestinal tract. Korean J Gastroenterol. 1998; 31:598–604.
29. Kim JP, Kwon OJ, Shim HS, Kim RB, Kim JH, Woo SH. Analysis of clinical feature and management of fish bone ingestion of upper gastrointestinal tract. Clin Exp Otorhinolaryngol. 2015; 8:261–267.

30. Chung H, Kwak YH, Kim DK, Jung JY, Lee JH, Kim HB. Usefulness of plain radiographs for management of suspected fishbone impaction in digestive tract of children. J Korean Soc Emerg Med. 2012; 23:537–542.
31. Kim JE, Ryoo SM, Kim YJ, et al. Incidence and clinical features of esophageal perforation caused by ingested foreign body. Korean J Gastroenterol. 2015; 66:255–260.

32. Sheth N, Diner WC. Swallowing problems in the elderly. Dysphagia. 1988; 2:209–215.

33. Evans RM, Ahuja A, Rhys Williams S, Van Hasselt CA. The lateral neck radiograph in suspected impacted fish bones: does it have a role? Clin Radiol. 1992; 46:121–123.
34. Gautam V, Phillips J, Bowmer H, Reichl M. Foreign body in the throat. J Accid Emerg Med. 1994; 11:113–115.

35. Ginsberg GG. Management of ingested foreign objects and food bolus impactions. Gastrointest Endosc. 1995; 41:33–38.

36. Mosca S, Manes G, Martino R, et al. Endoscopic management of foreign bodies in the upper gastrointestinal tract: report on a series of 414 adult patients. Endoscopy. 2001; 33:692–696.

37. Sugawa C, Ono H, Taleb M, Lucas CE. Endoscopic management of foreign bodies in the upper gastrointestinal tract: a review. World J Gastrointest Endosc. 2014; 6:475–481.

38. Telford JJ. Management of ingested foreign bodies. Can J Gastroenterol. 2005; 19:599–601.

39. Connolly AA, Birchall M, Walsh-Waring GP, Moore-Gillon V. Ingested foreign bodies: patient-guided localization is a useful clinical tool. Clin Otolaryngol Allied Sci. 1992; 17:520–524.

40. Luk WH, Fan WC, Chan RY, Chan SW, Tse KH, Chan JC. Foreign body ingestion: comparison of diagnostic accuracy of computed tomography versus endoscopy. J Laryngol Otol. 2009; 123:535–540.

41. Ambe P, Weber SA, Schauer M, Knoefel WT. Swallowed foreign bodies in adults. Dtsch Arztebl Int. 2012; 109:869–875.

42. ASGE Standards of Practice Committee, Ikenberry SO, Jue TL, et al. Management of ingested foreign bodies and food impactions. Gastrointest Endosc. 2011; 73:1085–1091.

43. Ritchie T, Harvey M. The utility of plain radiography in assessment of upper aerodigestive tract fishbone impaction: an evaluation of 22 New Zealand fish species. N Z Med J. 2010; 123:32–37.
44. Ngan JH, Fok PJ, Lai EC, Branicki FJ, Wong J. A prospective study on fish bone ingestion. Experience of 358 patients. Ann Surg. 1990; 211:459–462.
45. Wu IS, Ho TL, Chang CC, Lee HS, Chen MK. Value of lateral neck radiography for ingested foreign bodies using the likelihood ratio. J Otolaryngol Head Neck Surg. 2008; 37:292–296.
46. Shihada R, Goldsher M, Sbait S, Luntz M. Three-dimensional computed tomography for detection and management of ingested foreign bodies. Ear Nose Throat J. 2009; 88:910–911.

47. Liew CJ, Poh AC, Tan TY. Finding nemo: imaging findings, pitfalls, and complications of ingested fish bones in the alimentary canal. Emerg Radiol. 2013; 20:311–322.

48. Marco De Lucas E, Sádaba P, Lastra García-Barón P, et al. Value of helical computed tomography in the management of upper esophageal foreign bodies. Acta Radiol. 2004; 45:369–374.

49. Goh BK, Tan YM, Lin SE, et al. CT in the preoperative diagnosis of fish bone perforation of the gastrointestinal tract. AJR Am J Roentgenol. 2006; 187:710–714.

50. Young CA, Menias CO, Bhalla S, Prasad SR. CT features of esophageal emergencies. Radiographics. 2008; 28:1541–1553.

51. Takada M, Kashiwagi R, Sakane M, Tabata F, Kuroda Y. 3D-CT diagnosis for ingested foreign bodies. Am J Emerg Med. 2000; 18:192–193.

52. Foley MJ, Ghahremani GG, Rogers LF. Reappraisal of contrast media used to detect upper gastrointestinal perforations: comparison of ionic water-soluble media with barium sulfate. Radiology. 1982; 144:231–237.

53. Loh KS, Tan LK, Smith JD, Yeoh KH, Dong F. Complications of foreign bodies in the esophagus. Otolaryngol Head Neck Surg. 2000; 123:613–616.

54. Chaikhouni A, Kratz JM, Crawford FA. Foreign bodies of the esophagus. Am Surg. 1985; 51:173–179.
55. Gmeiner D, von Rahden BH, Meco C, Hutter J, Oberascher G, Stein HJ. Flexible versus rigid endoscopy for treatment of foreign body impaction in the esophagus. Surg Endosc. 2007; 21:2026–2029.

56. Tan S, Tan S, Peng M, Yu F. Management of an ingested fish bone in the lung using video-assist thoracic surgery: a case report. Medicine (Baltimore). 2015; 94:e943.
57. Li ZS, Sun ZX, Zou DW, Xu GM, Wu RP, Liao Z. Endoscopic management of foreign bodies in the upper-GI tract: experience with 1088 cases in China. Gastrointest Endosc. 2006; 64:485–492.

58. Zhang S, Wang J, Wang J, Zhong B, Chen M, Cui Y. Transparent cap-assisted endoscopic management of foreign bodies in the upper esophagus: a randomized, controlled trial. J Gastroenterol Hepatol. 2013; 28:1339–1342.

59. Lee JS, Chun HJ, Lee JM, et al. Salvage technique for endoscopic removal of a sharp fish bone impacted in the esophagus using a transparent cap and detachable snares. Korean J Gastroenterol. 2013; 61:215–218.

60. Singh B, Kantu M, Har-El G, Lucente FE. Complications associated with 327 foreign bodies of the pharynx, larynx, and esophagus. Ann Otol Rhinol Laryngol. 1997; 106:301–304.

61. Hokama A, Uechi K, Takeshima E, et al. A fish bone perforation of the esophagus. Endoscopy. 2014; 46 Suppl 1 UCTN:E216–E217.

62. Jeon SH, Han DC, Lee SG, Park HM, Shin DJ, Lee YB. Eikenella corrodens cervical spinal epidural abscess induced by a fish bone. J Korean Med Sci. 2007; 22:380–382.

63. Wei Y, Jahreiß L, Zhang Z, Albers AE. Acute airway obstruction due to retropharyngeal haematoma caused by a large fish bone in a patient with hypertension caused by a pheochromocytoma. BMJ Case Rep. 2015; 2015:bcr2014208644.

64. Sinha R, Sen I, Saha J, Mukherjee A, Guha R. Migration of a fish bone from the upper aerodigestive tract to the skin of the neck: a case report. Ear Nose Throat J. 2013; 92:E15.

65. Aslan M, Çelik Y, Dülger AC, et al. Submucosal hematoma of the esophagus due to fish bone ingestion. Turk J Gastroenterol. 2014; 25 Suppl 1:302–303.

66. Kim HG, Kim BH, Lim DJ, Kang SH. A case of removal of esophageal foreign body causing esophageal submucosal hematoma. Korean J Otorhinolaryngol-Head Neck Surg. 2007; 50:958–960.
67. Wu HC, Hsia JY, Hsu CP. Esophageal laceration with intramural dissection mimics esophageal perforation. Interact Cardiovasc Thorac Surg. 2008; 7:864–865.

68. Shim CS, Lee MS, Cho JY, et al. Esophagus, stomach & intestine: a case of tracheoesophageal fistula caused by fish bone induced trauma with complete healing by using the fibrinogen: thrombin glue. Korean J Gastrointest Endosc. 1997; 17:49–54.
69. Mukhopadhyay B, Tripathy BB, Saha S, Shukla RM, Saha SR, et al. Acquired tracheo-oesophageal fistula: a case report. J Indian Med Assoc. 2008; 106:806–808.
70. Pan S, Chai Y, Shen G. Recurrent pneumonia caused by a migrated esophageal foreign body. Thorac Cardiovasc Surg. 2013; 61:513–515.

71. Matsuda E, Okabe K, Takahagi A, et al. Pulmonary abscess caused by fish bone. Kyobu Geka. 2013; 66:219–222.
72. Kim YS, Lee SW, Kang SB, Nam SW, Lee DS, Park K. Esophageal perforation and empyema after fish bone swallowing. Korean J Gastrointest Endosc. 2007; 34:320–323.
73. Kim KM, Jang AS, Kim SW, et al. A case of acute mediastinitis associated with fish bone with successful conservative treatment. Tuberc Respir Dis. 2002; 53:344–348.

74. Choi EK, Kwon KH, Choi YW, Oh SK, Jeong JW, Park YK. A case of acute purulent pericarditis with pericardial performation by esophageal foreign body. J Korean Soc Echocardiogr. 2000; 8:247–251.

75. Liang H, Xu Z, Feng Q, Ma L. Recurrent cerebral embolism secondary to esophageal and atrial foreign body complicated by infective endocarditis. J Thorac Cardiovasc Surg. 2014; 148:e213–e214.

76. Blanco Ramos M, Rivo Vázquez JE, García-Fontán E, Amoedo TO. Systemic air embolism in a patient with ingestion of a foreign body. Interact Cardiovasc Thorac Surg. 2009; 8:292–294.

77. Kunishige H, Myojin K, Ishibashi Y, Ishii K, Kawasaki M, Oka J. Perforation of the esophagus by a fish bone leading to an infected pseudoaneurysm of the thoracic aorta. Gen Thorac Cardiovasc Surg. 2008; 56:427–429.

78. Macchi V, Porzionato A, Bardini R, Parenti A, De Caro R. Rupture of ascending aorta secondary to esophageal perforation by fish bone. J Forensic Sci. 2008; 53:1181–1184.

79. Sauer BM, Staritz M, Perdekamp MG. Lethal bleeding after accidental swallowing of a wooden meat skewer. Z Gastroenterol. 2009; 47:749–752.
80. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014; 7:183–197.
81. Sung HY, Lee IS, Kim SI, et al. Clinical features of abdominal actinomycosis: a 15-year experience of a single institute. J Korean Med Sci. 2011; 26:932–937.

82. Kikuchi K, Tsurumaru D, Hiraka K, Komori M, Fujita N, Honda H. Unusual presentation of an esophageal foreign body granuloma caused by a fish bone: usefulness of multidetector computed tomography. Jpn J Radiol. 2011; 29:63–66.

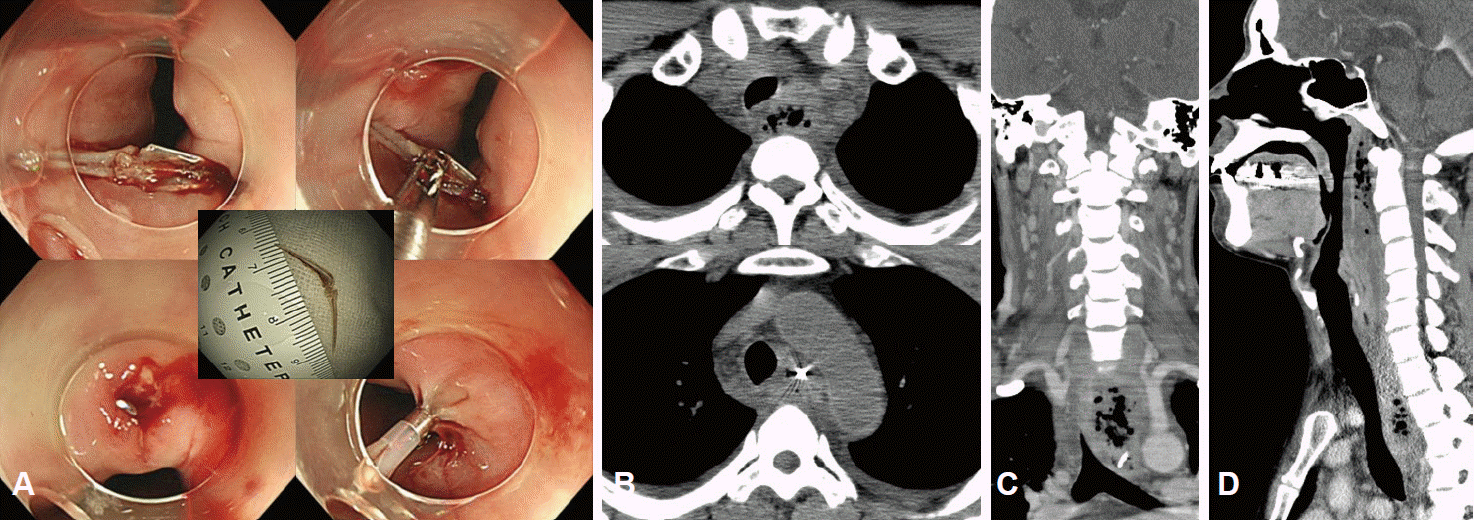
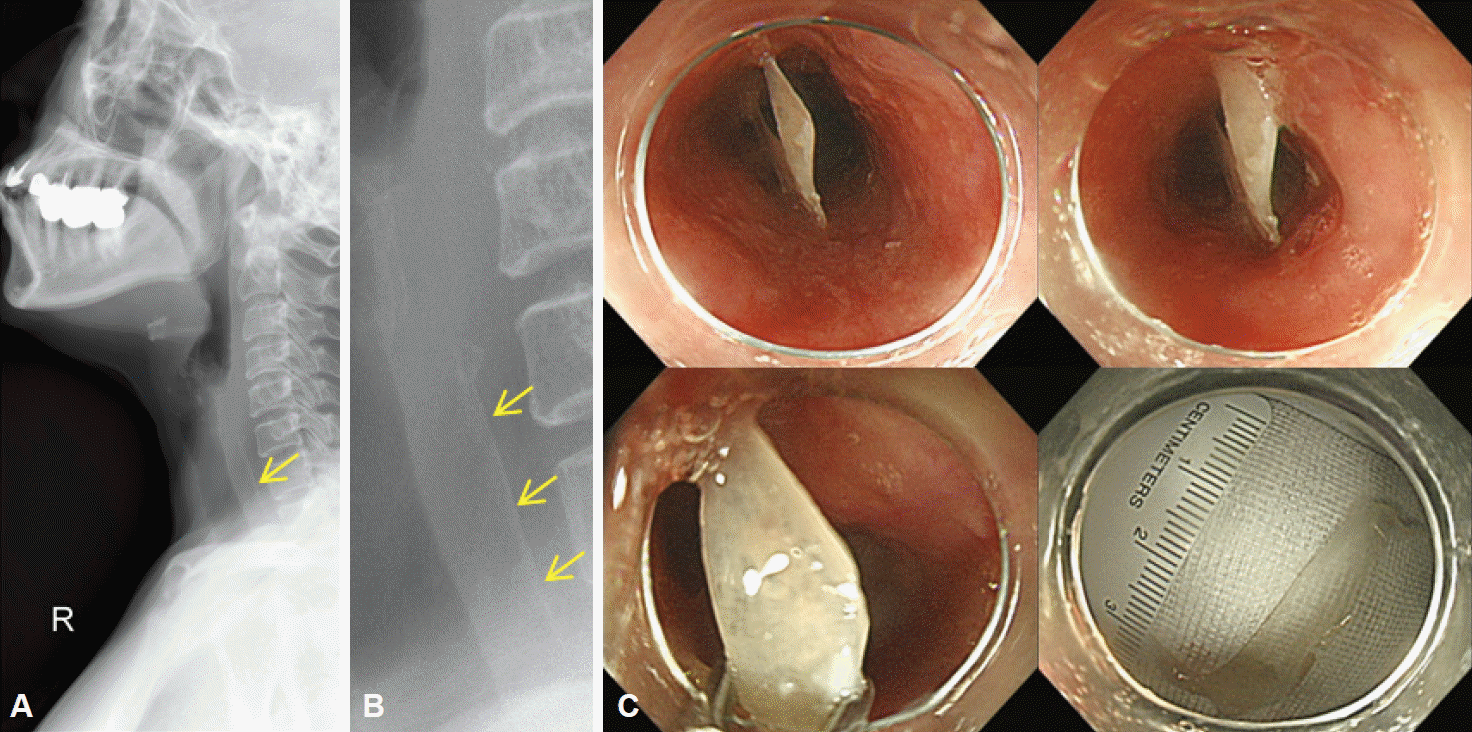
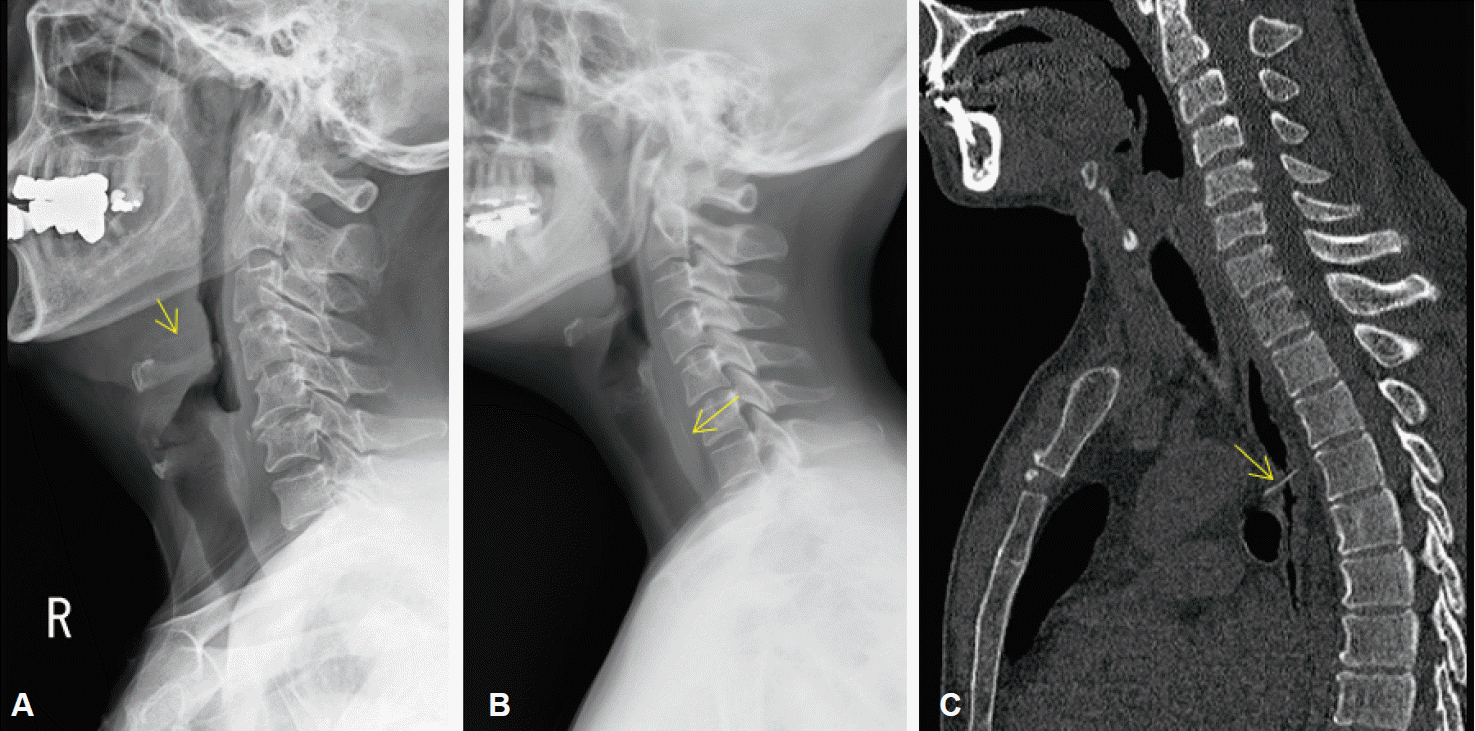
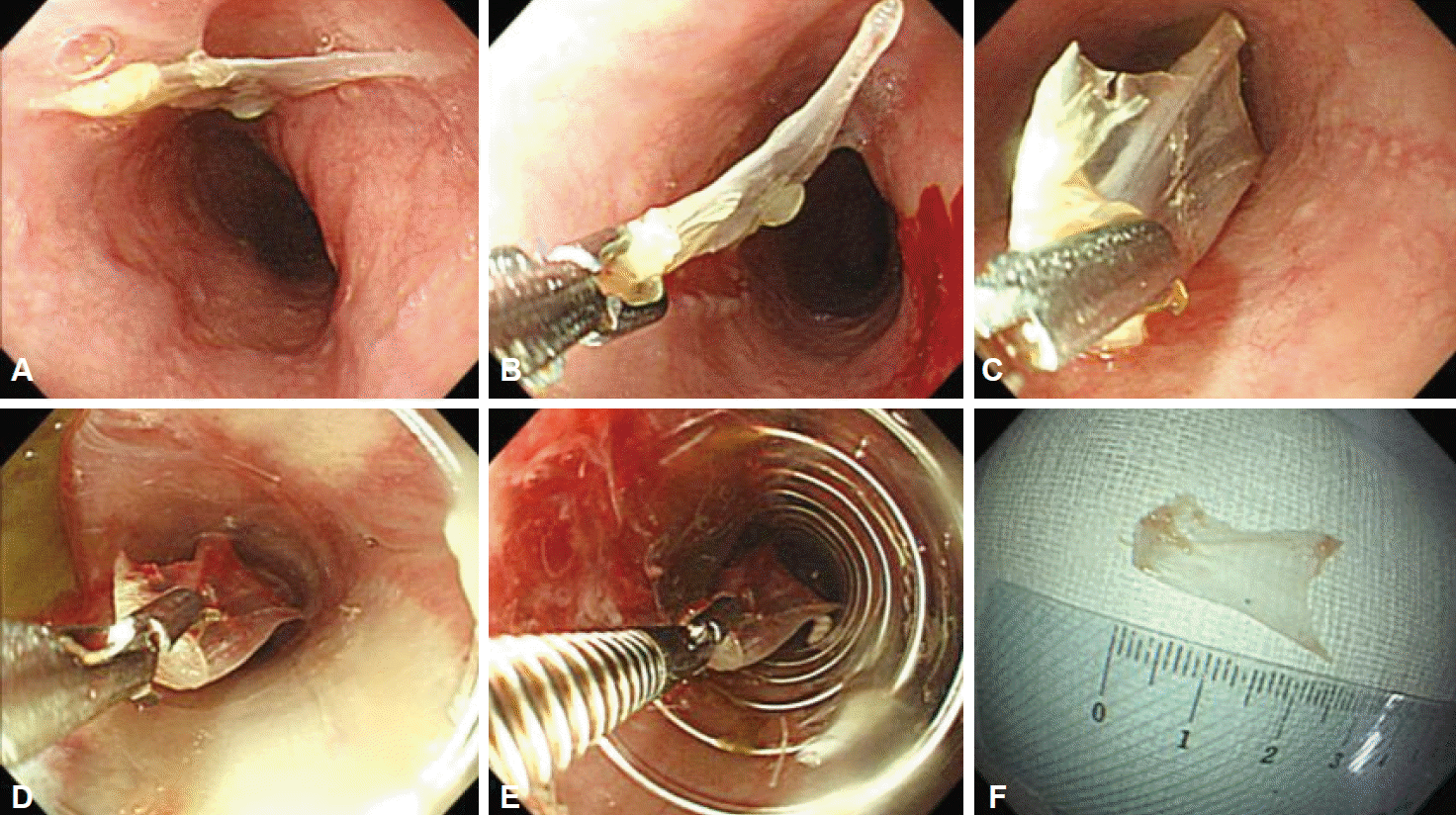




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download