Abstract
Early diagnosis of Borrmann type 4 advanced gastric cancer (AGC) is very important for improving the prognosis of AGC patients. Because there is no definite mass in most cases of Borrmann type 4 AGC, its accurate diagnosis via endoscopy requires an understanding of its pathogenesis and developmental process. Moreover, many people confuse linitis plastica (LP) type gastric cancer (GC), scirrhous GC, and Borrmann type 4 AGC. To distinguish each of these cancers, knowledge of their endoscopic and pathological differences is necessary, especially for LP type GCs in the developmental stage. In conclusion, diagnosis of pre-stage or latent LP type GC before progression to typical LP type GC requires the detection of IIc-like lesions in the fundic gland area. It is also crucial to identify any abnormalities such as sclerosis of the gastric wall and hypertrophy of the mucosal folds during endoscopy.
Qualitative diagnosis of advanced gastric cancer (AGC) is often a major problem for endoscopists. Owing to the absence of a definite ulcer or mass lesion, Borrmann type 4 AGC is overlooked in endoscopic examinations in many cases. Even when diagnosed, surgery is no longer feasible in most cases. Hence, early diagnosis is crucial for improving the prognosis of patients with this disease [1].
Borrmann type 4 AGC is often thought to be synonymous with linitis plastica (LP) type gastric cancer (GC) or scirrhous GC; however, these cancers are not the same [2,3]. In this article, we properly define each of these cancer types and analyze the differences among them. From the perspective of the endoscopist, it is helpful to consider Borrmann type 4 AGC as scirrhous GC in order to understand its diagnosis and pathology. To briefly explain these similar cancer types, we present a Venn diagram and examples in Fig. 1.
In this review, we first discuss the developmental process of LP type GCs, which originate in the fundic gland and then form on the gastric body. We next describe the features of each developmental step in order to aid early diagnosis. Lastly, we examine the endoscopic diagnosis of LP type GC and its differentiation from similar lesions.
AGC was first classified in 1926 by Borrmann [4], who defined type 4 AGC as follows: “diffuse cancer, ulcers are almost invisible, boundary is not clear even in the resected stomach.” However, even if ulcerative lesions are present, a case is classified as Borrmann type 4 AGC if a large diffuse infiltrating cancerous tumor is observed (Fig. 2).
The term “scirrhous” refers to the quality of the cancer at the gross and tissue level, rather than the quantity of the diffused area or the gross type on endoscopy. It describes localized quality in regard to the histologic findings of the cancer, and therefore, is a pathological term that applies to a wide range of tumors with rigid fibrous proliferation. Scirrhous GCs have extensive areas of pronounced fibrous hyperplasia and typically display significant luminal narrowing (Fig. 3). However, from the macroscopic viewpoint in endoscopy, scirrhous GC refers not only to limited or IIc-like AGCs, but also to lesions with extensive infiltration. Although Borrmann type 4 AGC and scirrhous GC are currently considered to be nearly the same entity in most cases, there are differences between them. The main characteristic of scirrhous GC is sclerosis or induration, while that of Borrmann type 4 AGC is diffuse distribution (Fig. 4) [5,6].
The term “LP” indicates thickening and sclerosis of the gastric body due to the growth of fibrous tissue, which was first misdiagnosed as inflammatory changes similar to the belt-like shape described by Brinton et al. [7] in 1865. It was then used to describe a specific type of stomach cancer, and its current meaning is almost the same as that of scirrhous [8]. LP is used interchangeably with leather bottle stomach, cirrhosis of the stomach, fibroid induration, fibromatosis of the stomach, gastric sclerosis, and gastritis granulomatosa fibroplastica. However, in some cases, good distensibility is maintained even though the cancer has invaded all layers of the gastric wall. Such cases could be diagnosed as Borrmann type 4 AGC or superficial extensive GC but are not as scirrhous GC owing to the presence of distensibility [2].
Strictly speaking, Borrmann type 4 AGCs differ from LP type GCs in terms of gross findings, the primary site, and the clinical course. In particular, LP type GCs develop mainly in the upper body without involvement of the lesser curvature, and sometimes exhibit intramucosal extension rather than spreading throughout the submucosal layer. Moreover, many cases of LP type GC involve diffuse spreading below the submucosal layer of the entire stomach, and therefore, meandering hypertrophic mucosal folds are widely observed (Fig. 5). On the other hand, Borrmann type 4 AGC is commonly confined to the pyloric antrum (Fig. 4), and its extent is usually similar in the mucosal and submucosal layers. Therefore, while LP type GCs involve diffuse spreading into all layers, Bormann type 4 AGCs are typically confined to a specific area (e.g., the antrum) and do not contain meandering hypertrophic mucosal folds [9].
Scirrhous GC can be grossly classified as a pyloric stenosis that occurs in the pyloric gland area or a leather bottle-shaped deformity in the fundic gland of the upper body. We refer to the second type as “LP type GC” in this review.
LP type GC generally has a poor prognosis despite the recent development of diagnostic techniques for stomach cancer. The main reasons for this poor prognosis are the difficulty of early detection and the unique developmental process of this cancer.
Because endoscopy typically does not detect abnormalities until several months after disease onset, it is not uncommon to diagnose LP type GC at the unresectable or advanced stage. Most research on the occurrence and development of LP type GC consists of retrospective studies performed in Japan [6]. Although the initial stages of LP type GC have been relatively well studied, the events occurring during its progression and development remain largely unknown.
LP type GC was defined by Sugiyama et al. [10] in 1980 as a lesion infiltrating twice as prolifically into the submucosa compared with the mucosa, with no primary localized mass formation. It was later divided into three clinical stages [11]: typical (defined as submucosal infiltration of a tumor occupying more than 1/4 of the total stomach with narrowing of the gastric lumen), latent (defined as tumor invasion of more than 1/4 of the total area without luminal narrowing), and pre-LP (defined as cancer cells invading less than 1/4 of the total area of the submucosal infiltration and as the absence of luminal stenosis) [11].
In 1980, Nakazawa et al. [12] identified two distinct courses in the progression of LP type GCs: slow progression (slow phase) and fast progression (fast phase). Takeda et al. [13] retrospectively described 16 cases of LP type GCs. According to their results, progression to LP type GC required 21 months if the first lesion was ulcerative. If the presenting lesion was a IIc lesion without ulceration (Fig. 6), progression to LP type GC also required a long time. In contrast, if only localized mucosal edema was noted, LP type GC developed in 12 to 21 months. Moreover, if the lesion had giant mucosal folds, it quickly advanced to LP type GC within 10 months.
Ohgushi et al. [14] described the progression of seven LP type GCs in a retrospective study. Initially, the primary tumors resembled IIc-like lesions in the fundic gland area or transition zone. After formation of the IIc lesions, the surrounding mucosa became sclerotic, with a flat elevated appearance. Eventually, the lesions became malignant ulcers and gradually invaded the submucosal layer, even if the ulcers were healed.
In 1992, Takizawa [15] surveyed 245 patients with LP type GCs over a 10-year period. The primary lesion occurred in the fundic gland in all patients. Pathologically, most lesions were diagnosed as undifferentiated carcinomas, and lymphatic metastasis was noted in more than 50% of the cases. Observation of early gastric cancers (EGCs) with IIc-like lesions revealed that these lesions progressed to pre-stage LP type GC. Compared with other types of advanced GCs, patients with LP type GC were younger at disease onset and more frequently women.
A retrospective study performed a histological analysis of the development of LP type GCs (Fig. 7) [16]. Initially, undifferentiated carcinomas arose from the fundic gland mucosa, presenting as approximate 2 cm or smaller IIc or IIb lesions without ulceration. After formation of the IIc lesions, the cancer cells infiltrated the submucosal layer, forming LP type GCs. Progression to LP type GC with submucosal involvement typically required 2 or 3 years. After progression, the cancer cells usually invaded another layer of the gastric wall. Based on observations of the invasion of the mucosal layer, some cases had ulcerative lesions at the primary site.
Cancer invasion of a layer is accompanied by fibrous tissue formation. During this period, enlargement of the mucosal folds, IIc-like lesions, and partial deformity of the gastric wall can occur. Owing to the contraction in the progression of time, fibrous tissue typically causes luminal narrowing. At this point, the cancer is usually detected as a luminal narrowing of the stomach or a leather bottle-like morphology. Six or more years have often passed since presentation of the initial lesion. As previously mentioned, the transition from pre-stage to typical LP type GC can take 1 to 3 years; hence, the target lesion for early detection of LP type GC is a less than 2 cm-sized IIc lesion without ulceration or abnormal fold convergence in the upper body [11].
LP type GCs account for approximately 10% to 20% of all GCs [2], and most patients do not survive more than 5 years after diagnosis. In Japan, LP type GCs account for approximately 12.3% to 16.3% of all AGCs. However, in recent years, the incidence of LP type GC has fallen. Because of advancements in diagnostic techniques and increased understanding of the endoscopic characteristics of LP type GC, diagnosis of IIc type EGC of the fundic gland has increased, which might explain the reduced overall incidence of LP type AGC [17].
LP type GCs rapidly progress and have a poor prognosis. Most rapidly progressing GCs are LP type GCs. Because upper endoscopy and upper gastrointestinal series do not show abnormal findings until several months after disease onset, LP type GCs are often diagnosed at an unresectable or advanced stage [18]. Moreover, diagnosis of LP type GC in initial examinations is typically difficult for two major reasons. First, the cancer cells are mostly diffusely distributed in the submucosal layer or a deeper layer (the proper muscle or serosal layer). Because of the atypical distribution, the exposed mucosal lesion of the cancer is much smaller than the actual invasion area of the lesion. Second, as previously mentioned, the development of LP type GC involves two different stages. Moreover, contraction and sclerosis of the gastric wall occur in a relatively short period of time during the progression of LP type GCs. Therefore, in general, the clinical diagnosis of LP type GC is very difficult before the loss of distensibility or appearance of structural deformities due to sclerosis or narrowing [19]. On the other hand, LP type GCs are known to occur relatively more often in women and at a young age. The typical age at disease onset is 20 to 40 years in women and 60 years in men [6].
Scirrhous GCs can be grossly categorized as giant-fold type (body type) and flat type (LP type, non-giant-fold type, antral type). In the USA, the more common type is the flat type, in which the gastric mucosa atrophies and undergoes diffuse wall thickening in a large portion of the stomach. This type mainly involves the antrum and lesser curvature side without hypertrophy of the mucosal folds (giant folds). In Korea and Japan, the giant-fold type is more common. This type involves large fold changes accompanied by hypertrophy, sclerosis, nodularity, and thickening of the stomach body and greater curvature side, although there are no atrophic changes.
According to the study by Nakamura [16], which examined the early-stage endoscopic findings for LP type GCs, incipient lesions in the fundic gland can be detected as IIc-like lesions less than 2 cm in size without hypertrophy of the mucosal folds or fold convergence. However, it is very difficult to diagnose early-stage LP type GC in the absence of abnormal distensibility (Fig. 6).
A review of LP type GCs led to two conclusions. First, although the final stage of sclerosis of the gastric wall occurs within a short amount of time, the period from development to detection of LP type GCs is not short. Second, it is not easy to detect the initial lesion before sclerosis of the gastric wall, and physicians must analyze all small lesions in cases of gastritis, especially small atypical erosions (IIc-like lesions without fold convergence on the posterior wall or greater curvature side), as well as those in the normal fundic gland area. These IIc lesions are thought to be the initial point, spreading in association with infiltration of the deep layer in the early stage and producing abnormal elevations around themselves, while accompanied by broad, rapid infiltration of the submucosal layer [11]. However, it can be difficult to distinguish pre- or latent LP type GCs from other types of gastritis with IIc-like lesions.
Typical endoscopic findings for scirrhous GCs include fold changes with hypertrophy, sclerosis, nodularity, and thickening. Pathologically, there are hypertrophic changes in the fundic gland in the giant-fold type. On the other hand, in the flat type, mucosal atrophic changes are more common, with diffuse wall thickening in each layer and no fold hypertrophy. Most cases involve a mixture of the giant and flat types.20
Based on the sites of cancer cell invasion, main involvement of the stomach body and greater curvature often indicates the giant-fold type, whereas the flat type is primarily confined to the antrum and lesser curvature side. The endoscopic findings for typical LP type GCs are hypertrophic changes, convergence of the folds, and blurring of the demarcation between the fold and the mass, with irregular elevated lesions. Because a lesion often contains a small erosion or ulcer on its surface, its presence might be required for differential diagnosis of type IIc or III EGCs (Fig. 5). Despite wide submucosal invasion, most lesions are covered with normal mucosa. Therefore, redness or inflammation of the mucosa is often mistaken for superficial gastritis. Nevertheless, such lesions can usually be distinguished by narrowing of the lumen due to poor extensibility, which does not increase during air inflation [8].
Consequently, if there is poor distensibility or hypertrophy of the mucosal folds of the stomach during endoscopy, LP type GC should be considered. Although a histologic diagnosis is essential when LP type GC is suspected, mucosal biopsies often yield negative results because of the small size of the exposed mucosal lesion of the cancer. Therefore, in cases with suspicious endoscopic findings, a second biopsy should be performed after a short follow-up period (Fig. 8) [21-23]. Endoscopy shows normal mucosa in about 30% of cases. Because LP type tumors do not always cause mucosal damage, it can be difficult to clearly determine the boundaries of the actual normal mucosa tissue and the infiltrating tumor cells. In such cases, endoscopic findings such as the shelf effect or the absence of mucosal tenting caused by the infiltration of tumor cells could help do so [24].
Endoscopic ultrasonography (EUS) findings for LP type GCs include initial thickening of the mucosa. As the disease progresses, the mucosa gradually becomes indistinct, and thickening can be observed around the submucosa and proper muscular layer. The most important finding is that the muscle thickness increases to more than four times that of a normal muscle layer with inhomogeneous echogenicity [25].
EUS findings might aid differential diagnosis in cases in which endoscopy is not useful. For example, EUS can show thickening of the mucosal and submucosal layers in patients with gastric lymphomas or hypertrophic gastritis. However, it may also show thickening of other layers of the gastric wall during the progression of gastric lymphoma. Moreover, via EUS, mucosal thickening can be seen in Menetrier disease and submucosal thickening in anisakiasis [26].
Diffuse gastric diseases that widely spread throughout the stomach have hypertrophic mucosal folds and sclerotic gastric walls, as seen in scirrhous GCs. However, the specific characteristics of each disease allow them to be relatively easily differentiated.
Like scirrhous GCs, many diseases involve the formation of giant folds (fold convergence) such as those with normal giant folds, hypertrophic gastritis, Menetrier disease, Zollinger-Ellison syndrome, gastric lymphoma, and sarcoidosis, and inflammatory diseases including gastric syphilis, gastric tuberculosis, anisakiasis, eosinophilic gastritis, and gastritis cystic polyposa [9]. Differential diagnosis of diffuse gastric diseases, as based on sclerosis of the gastric wall and hypertrophy of the mucosal folds, is shown in Table 1.
Induration of the stomach is confirmed in an extension state in an upper gastrointestinal series using a blowing agent and endoscopic distension with air inflation. If the stomach is not fully inflated because of induration, repeated belching will occur during the endoscopic examination, requiring it to be discontinued. Therefore, radiologic examination with an upper gastrointestinal series is more useful for studying the overall shape or appearance of the lumen. If distension of the stomach lumen is poor, the physician should consider the possibility of diffuse sclerosis of the stomach and scirrhous GC. Scirrhous GCs have primary lesions with a depressed mucosa and are generally diagnosed as undifferentiated cancers based on pathologic findings. However, the gastroenterologist should be aware of misdiagnosis, because several reported cases showed no cancer cells on pathologic examination.
During scar or band formation in the healing stage of corrosive gastritis, sclerosis of the mucosal folds can occur owing to diffuse fibrosis of the submucosal layer. Sclerosis is also sometimes caused by invasion of pancreatic cancer cells or inflammation-related adhesion of the serosal layer after acute pancreatitis. In addition, failed distension of the stomach is observed in inflammatory diseases such as gastric syphilis [27] and Crohn disease. Autoimmune gastritis resulting in marked atrophy of the gastric mucosa, which is a very rare condition, can also present with diffuse sclerosis.
If the expansion of the stomach is relatively good or slightly poor and the gastric wall is soft and smooth, the physician should consider the possibility of malignant lymphoma, hypertrophic gastritis (Menetrier disease), or acute gastritis. After treatment for malignant gastric lymphoma or recovery from an acute inflammatory state, the expansion capacity of the gastric wall will normalize. However, in cases of acute corrosive gastritis, abnormal expansion could represent progressive induration of the gastric wall [28].
Giant fold changes in the stomach can appear owing to extensive infiltration of the tumor in malignant diseases and edema in benign or inflammatory diseases (Table 2). Giant fold changes are a typical endoscopic finding for scirrhous GCs. Because of the contraction of the stomach caused by fibrous proliferation, the folds of the stomach become much larger, with a width greater than 1 cm. The fold has a cerebral rotation shape because of flexion meandering, and wrinkles between the folds are observed as the folds become narrower or buried [29].
Although this fold change can be observed in malignant lymphomas and inflammatory diseases, the expansibility of the stomach is maintained, and each fold can be clearly observed owing to the retention of the gaps among the folds. In addition, the folds are round in shape and smooth [9]. Malignant lymphoma and hypertrophic gastritis (Menetrier disease) involve thickening of the folds around the stomach body [30], while acute gastritis involves thickening of the folds around the antrum. However, fold thickening is usually normalized after treatment.
Early diagnosis of Borrmann type 4 AGC is essential for improving the prognosis of AGC patients. Because there is no definite mass in most cases of Borrmann type 4 AGC, its accurate diagnosis via endoscopy requires an understanding of its pathogenesis and developmental process.
In addition, an understanding of the pathologic findings and endoscopic features of diffuse gastric diseases will allow physicians to distinguish Borrmann type 4 AGC from inflammatory diseases or malignant lymphomas with similar endoscopic findings.
Above all, diagnosis of pre-stage or latent LP type GC before progression to typical LP type GC requires the identification of IIc-like lesions in the fundic gland area. Detection of any abnormalities such as sclerosis of the gastric wall and hypertrophy of mucosal folds during endoscopy is also crucial.
REFERENCES
1. Kitamura K, Beppu R, Anai H, et al. Clinicopathologic study of patients with Borrmann type IV gastric carcinoma. J Surg Oncol. 1995; 58:112–117.

2. Shim CS. Easy to miss gastric cancer: Borrmann type 4. Paper presented at: The 19th Seminar of Korean Society of Gastrointestinal Endoscopy. 1998 Aug 30-Sep 3; Seoul, Korea.
3. Nishi M, Ichikawa H, Nakajima T, Maruyama K, Tahara E. Gastric Cancer. Tokyo: Springer;1993.
4. Borrmann R. Geschwülste des Magens und Duodenums. In : Borchardt H, Borrmann R, Christeller E, editors. Verdauungsschlauch: Erster Teil Rachen und Tonsillen, Speiseröhre Magen und Darm, Bauchfell. Vienna: Springer;1926. p. 812–1054.
5. Fuchigami T. Historical changes in gastric cancer: from the viewpoint of diagnosis. Stomach Intest. 2005; 40:11–17.
6. Park MI. The development of Linitis plastic gastric cancer and similar lesions. Paper presented at: The 13th Seminar of Korean Society of Gastrointestinal Endoscopy. 2006 Jul 9; Busan, Korea.
7. Brinton W; Harvard Medical School. Lectures on the Diseases of the Stomach: with an Introduction on Its Anatomy and Physiology. 2nd ed. Philadelphia (PA): Lea and Blanchard;1865.
8. Lee JH. Hypertrophic gastritis and Borrmann type IV. Korean J Gastrointest Endosc. 2010; 40(Suppl 1):S83–S85.
9. Lee DH. Early endoscopic finding of Borrmann type IV. Korean J Gastrointest Endosc. 2005; 30(Suppl 1):S81–S86.
10. Sugiyama N, Baba Y, Takagi K, Nakamura K. A study of the clinical course of linitis plastica type cancer of the stomach: comparison between the clinical course and the development curve of gastric cancer. Stomach Intest. 1980; 15:1153–1163.
11. Nakamura K, Kato Y, Misono T, et al. Growing process to carcinoma of linitis plastica type of the stomach from cancer-development. Stomach Intest. 1980; 15:225–234.
12. Nakazawa S, Kawaguchi S, Yoshino J, et al. Linitis plastica retrospective study for early detection. Stomach Intest. 1980; 15:1145–1151.
13. Takeda S, Suto Y, Kohli Y, et al. Initial findings and developments of linitis plastica type of gastric cancer. Stomach Intest. 1980; 15:1137–1144.
14. Ohgushi H, Yao T, Iwashita A. The primary foci of linitis plastics type of gastric cancer and their chronological changes. Stomach Intest. 1980; 15:1129–1136.
15. Takizawa T. Distinctive features of linitis plastica type gastric carcinoma with reference to lymphangiosis carcinomatosa. Stomach Intest. 1992; 27:591–598.
16. Nakamura K. Issues focused on the IIc lesion in adenocarcima of fundic gland area: from the landscape of “a way to linitis plastica”. Stomach Intest. 1987; 22:999–1001.
17. Shim CS. Medical approch for early stomach cancer detection. Paper presented at: Spring Symposim of Cancer of Seoul St. Mary’s Hospital of The Catholic University of Korea. 1996; Seoul, Korea.
18. Pedrazzani C, Marrelli D, Pacelli F, et al. Gastric linitis plastica: which role for surgical resection? Gastric Cancer. 2012; 15:56–60.

19. Hosokawa O, Kaizaki Y, Miyanaga T, et al. Endoscopic study of the early phase in linitis plastica type gastric gancer. Stomach Intest. 2008; 43:799–809.
20. Maruyama Y, Kageoka M, Nagata K, et al. Endoscopic diagnosis based on typical findings of scirrhous gastric cancer. Stomach Intest. 2010; 45:445–455.
21. Jeong HY. AGC Borrmann type IV cancer. Korean J Gastrointest Endosc. 2011; 43(Suppl 2):S215–S217.
22. Kim JO, Ryu CB, Cho JY, Lee JS, Lee MS, Shim CS. The usefulness of the endoscopic ultrasonography in the evaluation of the thickened gastric wall. Korean J Gastrointest Endosc. 2001; 22:139–145.
23. Ahn JB, Ha TK, Lee HR, Kwon SJ. An insufficient preoperative diagnosis of Borrmann type 4 gastric cancer in spite of EMR. J Gastric Cancer. 2011; 11:59–63.

24. Levine MS, Kong V, Rubesin SE, Laufer I, Herlinger H. Scirrhous carcinoma of the stomach: radiologic and endoscopic diagnosis. Radiology. 1990; 175:151–154.

25. Murata Y, Yamasaki T, Kitamura Y, et al. Diagnostic points of endoscopic ultrasonographic findings in scirrhous gastric cancer. Stomach Intest. 2010; 45:457–467.
26. Songür Y, Okai T, Watanabe H, Motoo Y, Sawabu N. Endosonographic evaluation of giant gastric folds. Gastrointest Endosc. 1995; 41:468–474.

27. Roh M, Sohn JH, Kim TY, et al. Gastric syphilis and membranous glomerulonephritis. Clin Endosc. 2015; 48:256–259.

28. Kamijo Y, Kondo I, Watanabe M, Kan’o T, Ide A, Soma K. Gastric stenosis in severe corrosive gastritis: prognostic evaluation by endoscopic ultrasonography. Clin Toxicol (Phila). 2007; 45:284–286.

29. Jee SR. Borrmann type 4 AGC. Paper presented at: The 46th Korean Society of Gastrointestinal Endoscopy Seminar. 2012 Mar 25; Goyang, Korea.
30. Chung IS, Park DH, Choi SW, et al. Esophagus, stomach & intestine: a case of Menetrier’s disease. Korean J Gastrointest Endosc. 1997; 17:167–172.
Fig. 1.
Relationship between diffuse gastric cancers (scirrhous gastric cancer, Borrmann type 4 advanced gastric cancer [AGC], and linitis plastica).
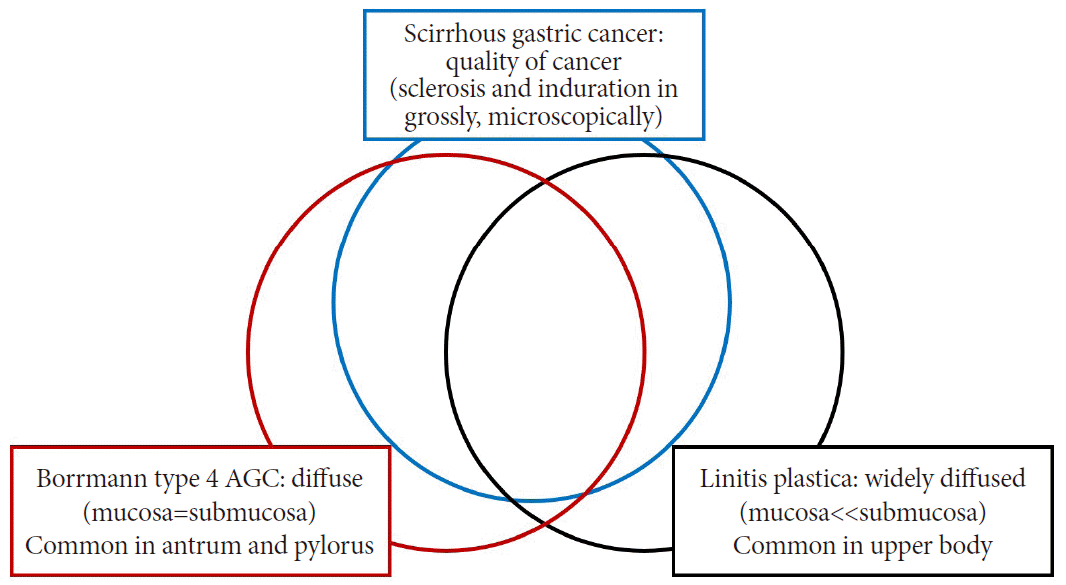
Fig. 2.
Typical case of Borrmann type 4 advanced gastric cancer. These images show similar findings with each diagnostic modality (A, B: endoscopy; C: positron emission tomography-computed tomography [CT]; D, E: abdominal CT). After total gastrectomy, the gross pathologic finding (F) also shows diffuse wall thickening, similar to that observed on CT.
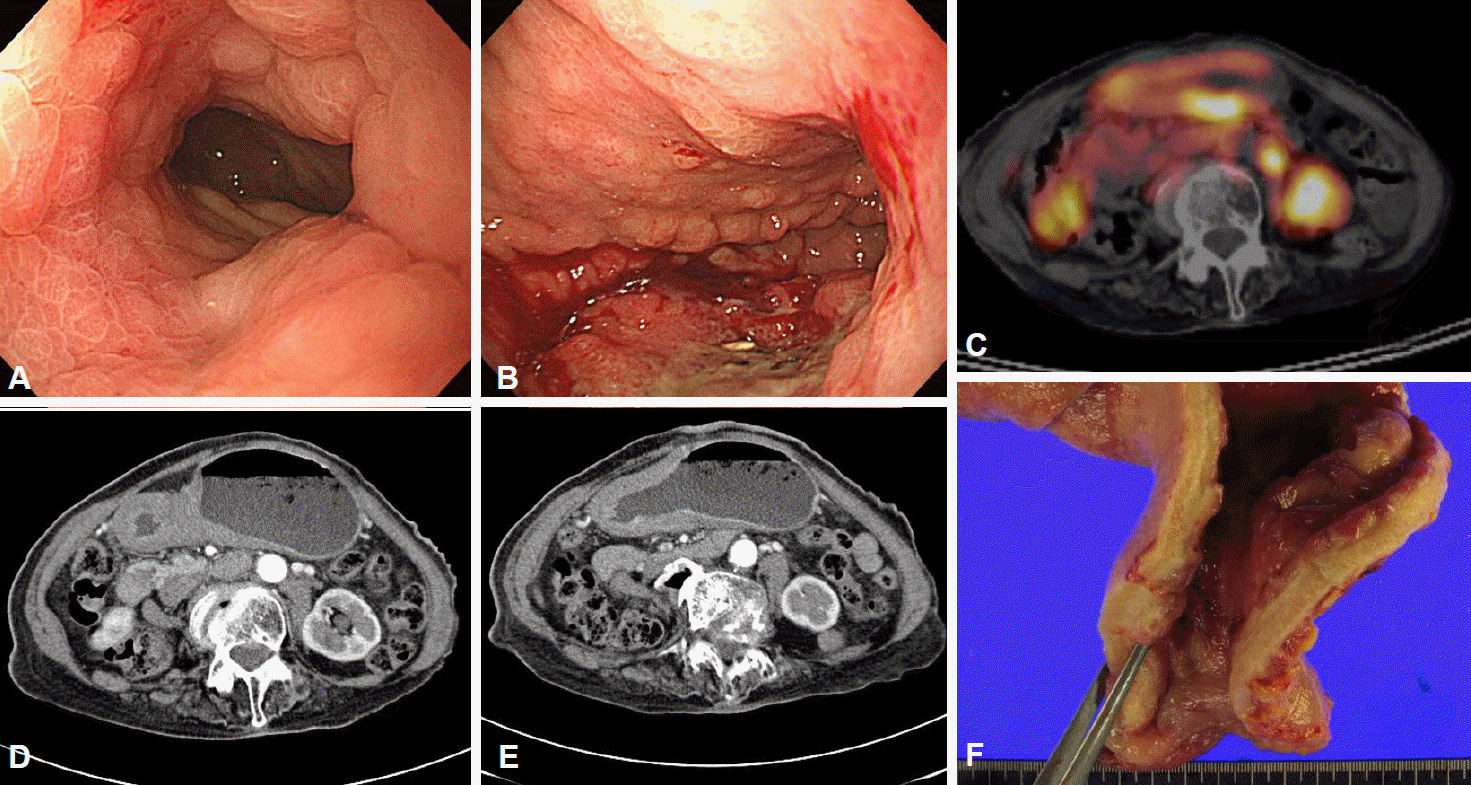
Fig. 3.
Examples of scirrhous gastric cancer. (A, B) Diffusely infiltrating masses with local areas of ulceration were present from the body to the distal antrum. (C, D) At the distal antrum, there were wide areas of extensive fibrous hyperplasia and significant luminal narrowing.
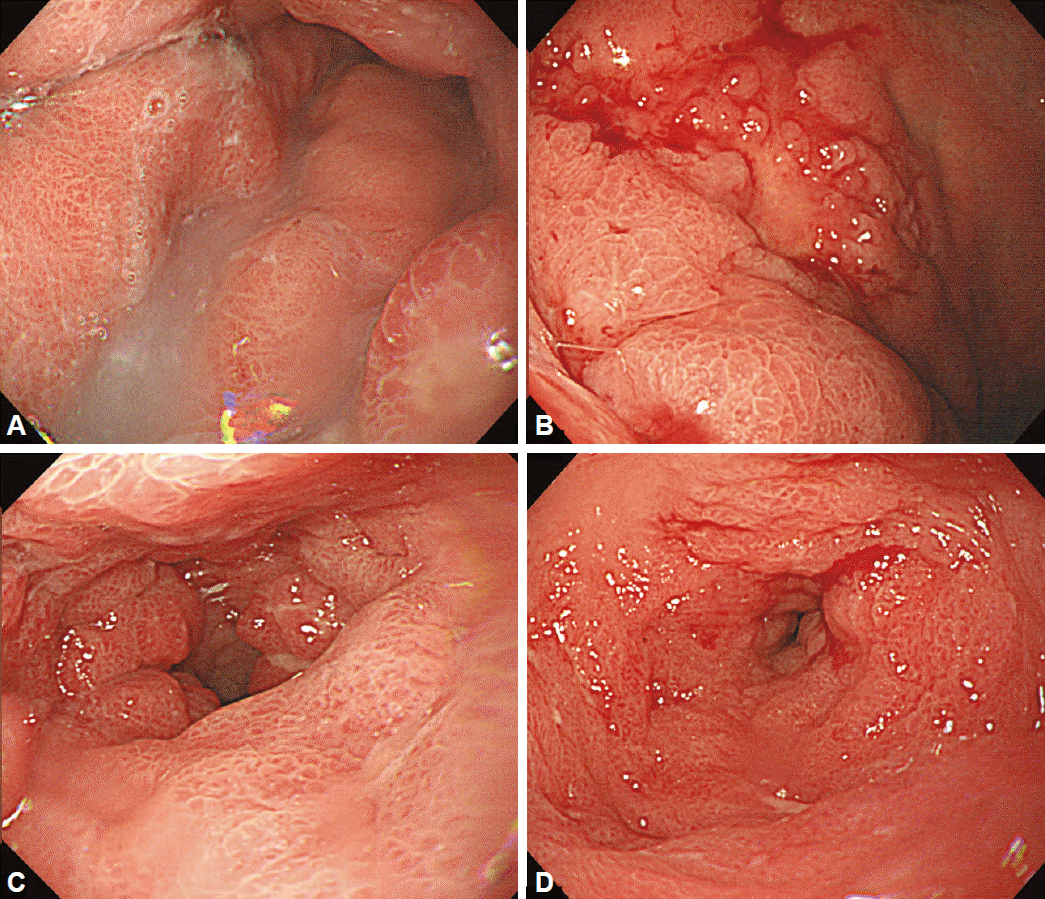
Fig. 4.
Examples of Borrmann type 4 advanced gastric cancer. (A, B) At the lesser curvature side of the proximal antrum to the lower body, diffuse, irregular, focal ulceration was observed. (C, D) Diffuse wall thickening and hypertrophy of the mucosal folds were present from the upper body to the antrum, with good distensibility.
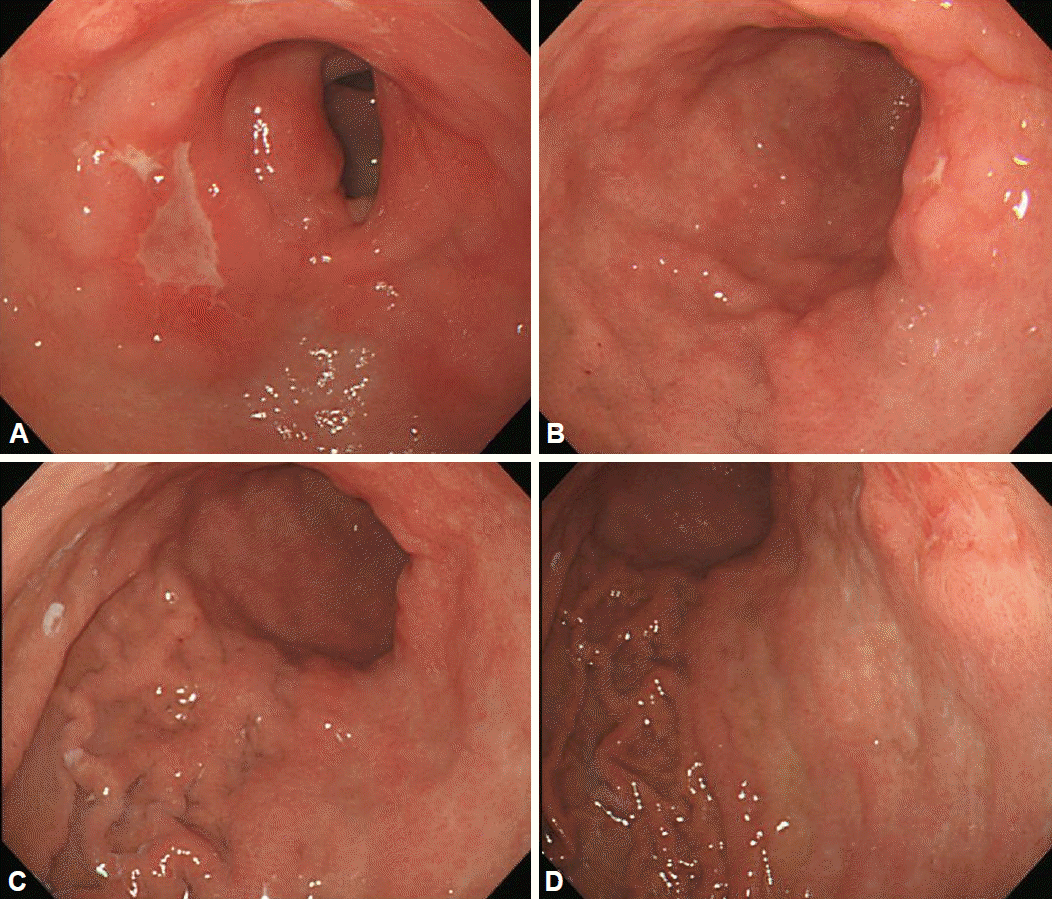
Fig. 5.
Examples of linitis plastica gastric cancer. (A, B) There was an ulcerative lesion on the greater curvature side of the upper body with sparing of the antrum. (C, D) Fold thickening with distensibility was observed on the upper to lower body.
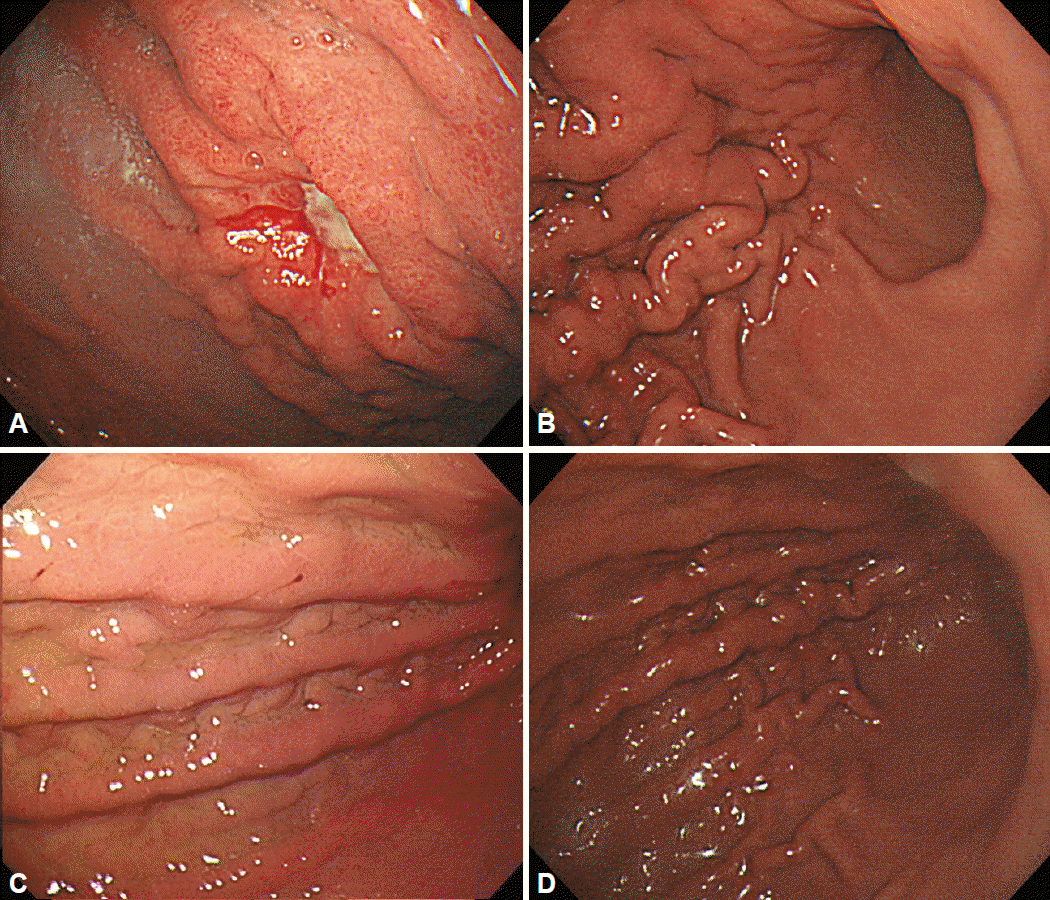
Fig. 6.
Morphologic changes in linitis plastic gastric cancer. Adapted from Nakamura, with permission from Igaku-Shoin Ltd [16].
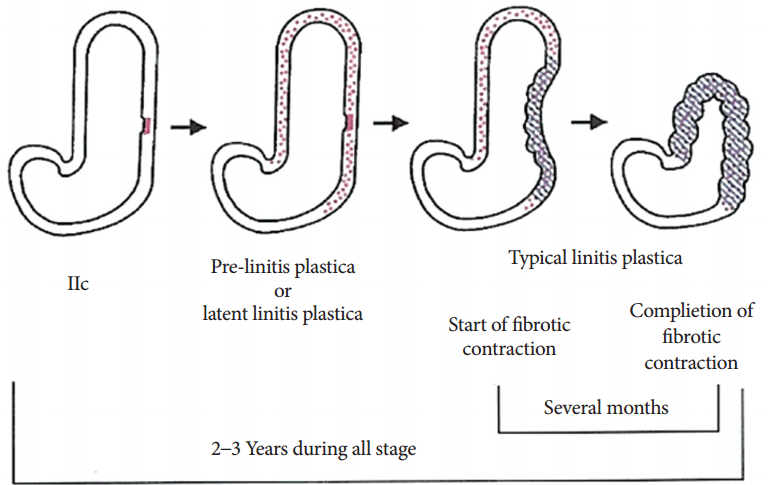
Fig. 7.
This picture shows an example of a IIc-like lesion (circle on image A). (A) A 50-year-old man was diagnosed with IIc-type gastric cancer on endoscopic biopsy. (B) Cutting shape of fold convergence was seen on endoscopy. (C, D) After chromoendoscopy with indigo carmine dye, the lesion could be observed more clearly. After gastrectomy, the main lesion was confirmed to be signet ring cell carcinoma without submucosal invasion.
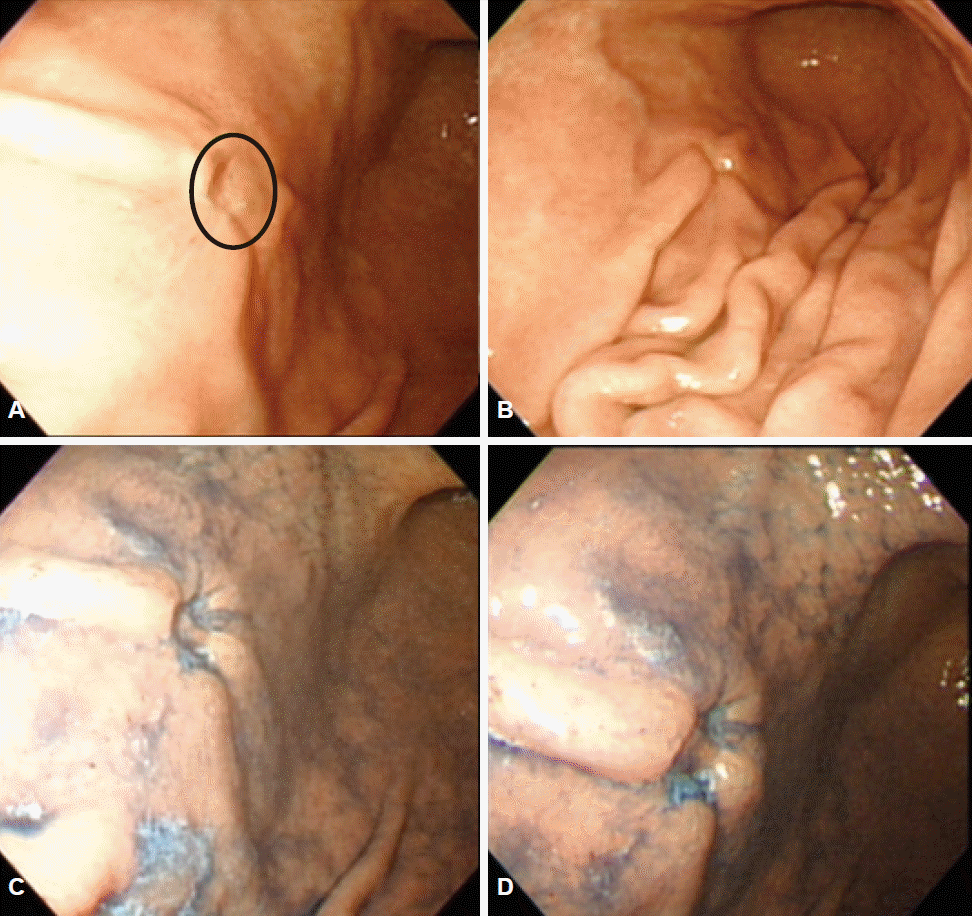
Fig. 8.
Summary of endoscopic findings and proposed diagnostic steps for scirrhous gastric cancer (GC). UGI, upper gastrointestinal; EUS, endoscopic ultrasound; CT, computed tomography; PET-CT, positron emission tomography-computed tomography; EMR, endoscopic mucosal resection.
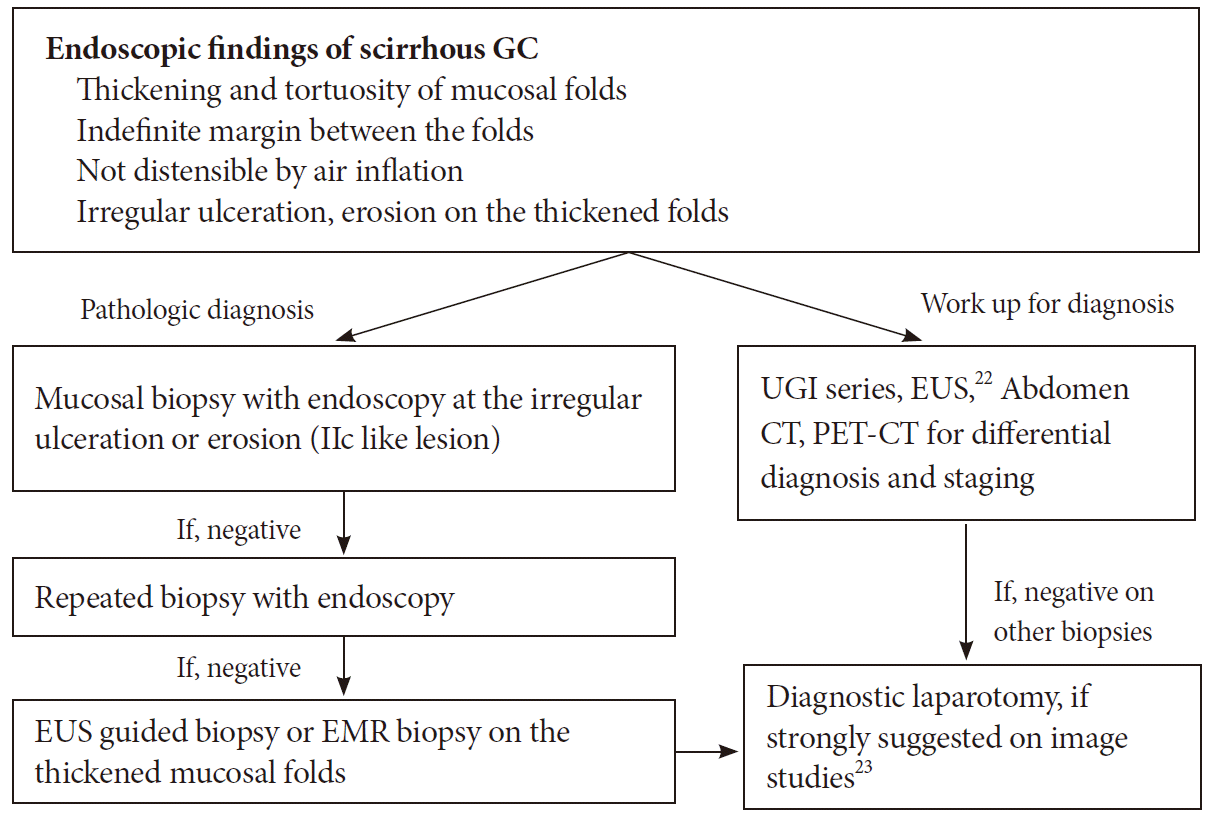
Table 1.
Differential Diagnosis of Gastric Diffuse Diseases
Table 2.
Inflammatory Disease Causing Giant Folds (Hypertrophy of Mucosal Folds)




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download