1. Irving JD, Adam A, Dick R, Dondelinger RF, Lunderquist A, Roche A. Gianturco expandable metallic biliary stents: results of a European clinical trial. Radiology. 1989; 172:321–326.

2. Dotter CT, Buschmann RW, McKinney MK, Rosch J. Transluminal expandable nitinol coil stent grafting: preliminary report. Radiology. 1983; 147:259–260.

3. Isayama H, Nakai Y, Toyokawa Y, et al. Measurement of radial and axial forces of biliary self-expandable metallic stents. Gastrointest Endosc. 2009; 70:37–44.

4. Loew BJ, Howell DA, Sanders MK, et al. Comparative performance of uncoated, self-expanding metal biliary stents of different designs in 2 diameters: final results of an international multicenter, randomized, controlled trial. Gastrointest Endosc. 2009; 70:445–453.

5. Lee JM, Lee SH, Chung KH, et al. Small cell- versus large cell-sized metal stent in endoscopic bilateral stent-in-stent placement for malignant hilar biliary obstruction. Dig Endosc. 2015; 27:692–699.

6. Saito H, Sakurai Y, Takamura A, Horio K. Biliary endoprosthesis using Gore-Tex covered expandable metallic stents: preliminary clinical evaluation. Nihon Igaku Hoshasen Gakkai Zasshi. 1994; 54:180–182.
7. Saleem A, Leggett CL, Murad MH, Baron TH. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc. 2011; 74:321–327.

8. Isayama H, Komatsu Y, Tsujino T, et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004; 53:729–734.

9. Telford JJ, Carr-Locke DL, Baron TH, et al. A randomized trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest Endosc. 2010; 72:907–914.

10. Kullman E, Frozanpor F, Soderlund C, et al. Covered versus uncovered self-expandable nitinol stents in the palliative treatment of malignant distal biliary obstruction: results from a randomized, multicenter study. Gastrointest Endosc. 2010; 72:915–923.

11. Bang BW, Jeong S, Lee DH, Lee JI, Lee SC, Kang SG. The biodurability of covering materials for metallic stents in a bile flow phantom. Dig Dis Sci. 2012; 57:1056–1063.

12. Kanasaki S, Furukawa A, Kane T, Murata K. Polyurethane-covered Nitinol Strecker stents as primary palliative treatment of malignant biliary obstruction. Cardiovasc Intervent Radiol. 2000; 23:114–120.
13. Maccioni F, Rossi M, Salvatori FM, Ricci P, Bezzi M, Rossi P. Metallic stents in benign biliary strictures: three-year follow-up. Cardiovasc Intervent Radiol. 1992; 15:360–366.

14. Miura S, Yoshioka T, Furuichi K, Tanaka T, Kichikawa K, Ohishi H. Mechanical properties of biliary metallic stents: an experimental comparison. Nihon Igaku Hoshasen Gakkai Zasshi. 2003; 63:201–209.
15. Dyet JF, Watts WG, Ettles DF, Nicholson AA. Mechanical properties of metallic stents: how do these properties influence the choice of stent for specific lesions? Cardiovasc Intervent Radiol. 2000; 23:47–54.

16. Duda SH, Wiskirchen J, Tepe G, et al. Physical properties of endovascular stents: an experimental comparison. J Vasc Interv Radiol. 2000; 11:645–654.

17. Jedwab MR, Clerc CO. A study of the geometrical and mechanical properties of a self-expanding metallic stent: theory and experiment. J Appl Biomater. 1993; 4:77–85.
18. Stoeckel D, Pelton A, Duerig T. Self-expanding nitinol stents: material and design considerations. Eur Radiol. 2004; 14:292–301.

19. Nuutinen JP, Clerc C, Tormala P. Theoretical and experimental evaluation of the radial force of self-expanding braided bioabsorbable stents. J Biomater Sci Polym Ed. 2003; 14:677–687.

20. Nakai Y, Isayama H, Kogure H, et al. Risk factors for covered metallic stent migration in patients with distal malignant biliary obstruction due to pancreatic cancer. J Gastroenterol Hepatol. 2014; 29:1744–1749.

21. Nakai Y, Isayama H, Kawakubo K, et al. Metallic stent with high axial force as a risk factor for cholecystitis in distal malignant biliary obstruction. J Gastroenterol Hepatol. 2014; 29:1557–1562.

22. Kawakubo K, Isayama H, Nakai Y, et al. Risk factors for pancreatitis following transpapillary self-expandable metal stent placement. Surg Endosc. 2012; 26:771–776.

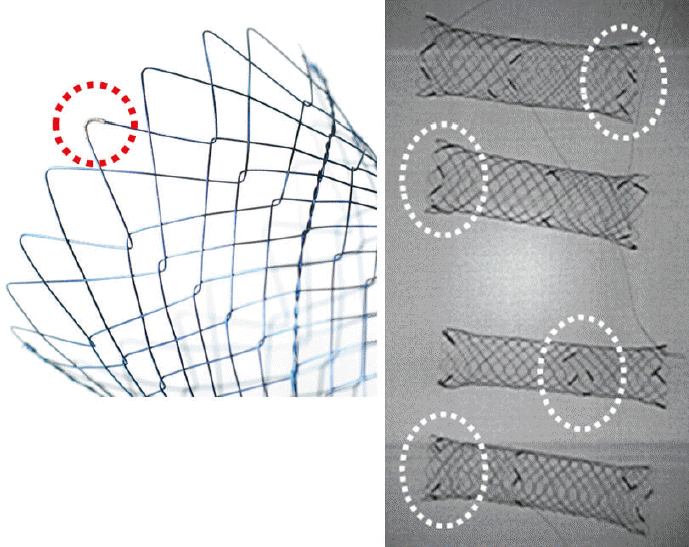
23. Isayama H, Nakai Y, Hamada T, Yamamoto N, Koike K. Development of an ideal self-expandable metallic stent design. Gastrointest Interv. 2015; 4:46–49.

24. Lee KJ, Chung MJ, Park JY, et al. Clinical advantages of a metal stent with an S-shaped anti-reflux valve in malignant biliary obstruction. Dig Endosc. 2013; 25:308–312.

25. Hu B, Wang TT, Wu J, Shi ZM, Gao DJ, Pan YM. Antireflux stents to reduce the risk of cholangitis in patients with malignant biliary strictures: a randomized trial. Endoscopy. 2014; 46:120–126.

26. Lee DH, Kang SG, Jeong S, et al. Local delivery system of immune modulating drug for unresectable adenocarcinoma: in vitro experimental study and in vivo animal study. Cardiovasc Intervent Radiol. 2006; 29:832–837.

27. Song TJ, Lee SS, Yun SC, et al. Paclitaxel-eluting covered metal stents versus covered metal stents for distal malignant biliary obstruction: a prospective comparative pilot study. Gastrointest Endosc. 2011; 73:727–733.

28. Jang SI, Kim JH, You JW, et al. Efficacy of a metallic stent covered with a paclitaxel-incorporated membrane versus a covered metal stent for malignant biliary obstruction: a prospective comparative study. Dig Dis Sci. 2013; 58:865–871.

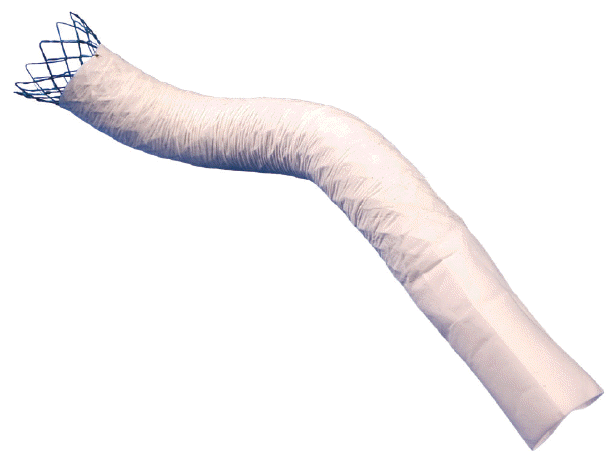
29. Isayama H, Kawabe T, Nakai Y, et al. Management of distal malignant biliary obstruction with the ComVi stent, a new covered metallic stent. Surg Endosc. 2010; 24:131–137.

30. Perri V, Boskoski I, Tringali A, et al. Prospective evaluation of the partially covered nitinol “ComVi” stent for malignant non hilar biliary obstruction. Dig Liver Dis. 2013; 45:305–309.

31. Park do H, Lee SS, Lee TH, et al. Anchoring flap versus flared end, fully covered self-expandable metal stents to prevent migration in patients with benign biliary strictures: a multicenter, prospective, comparative pilot study (with videos). Gastrointest Endosc. 2011; 73:64–70.
32. Moon SH, Kim MH, Park do H, et al. Modified fully covered self-expandable metal stents with antimigration features for benign pancreatic-duct strictures in advanced chronic pancreatitis, with a focus on the safety profile and reducing migration. Gastrointest Endosc. 2010; 72:86–91.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download