Abstract
Surgical resection is considered the standard of care in the management of symptomatic insulinoma. In this video, we describe the successful management of a symptomatic insulinoma by using linear array endoscopic ultrasound (EUS)-guided ethanol ablation in a poor surgical candidate. EUS-guided ethanol ablation of insulinoma offers a safer, effective, and less invasive alternative to surgery.
Insulinoma is the most common functional islet-cell tumor of the pancreas [1,2]. While surgical enucleation or resection is the standard of care, alternative management options may be mandated in symptomatic patients with unresectable disease, or for poor surgical candidates. We present a successful management of symptomatic insulinoma using endoscopic ultrasound (EUS)-guided ethanol ablation in a poor surgical candidate.
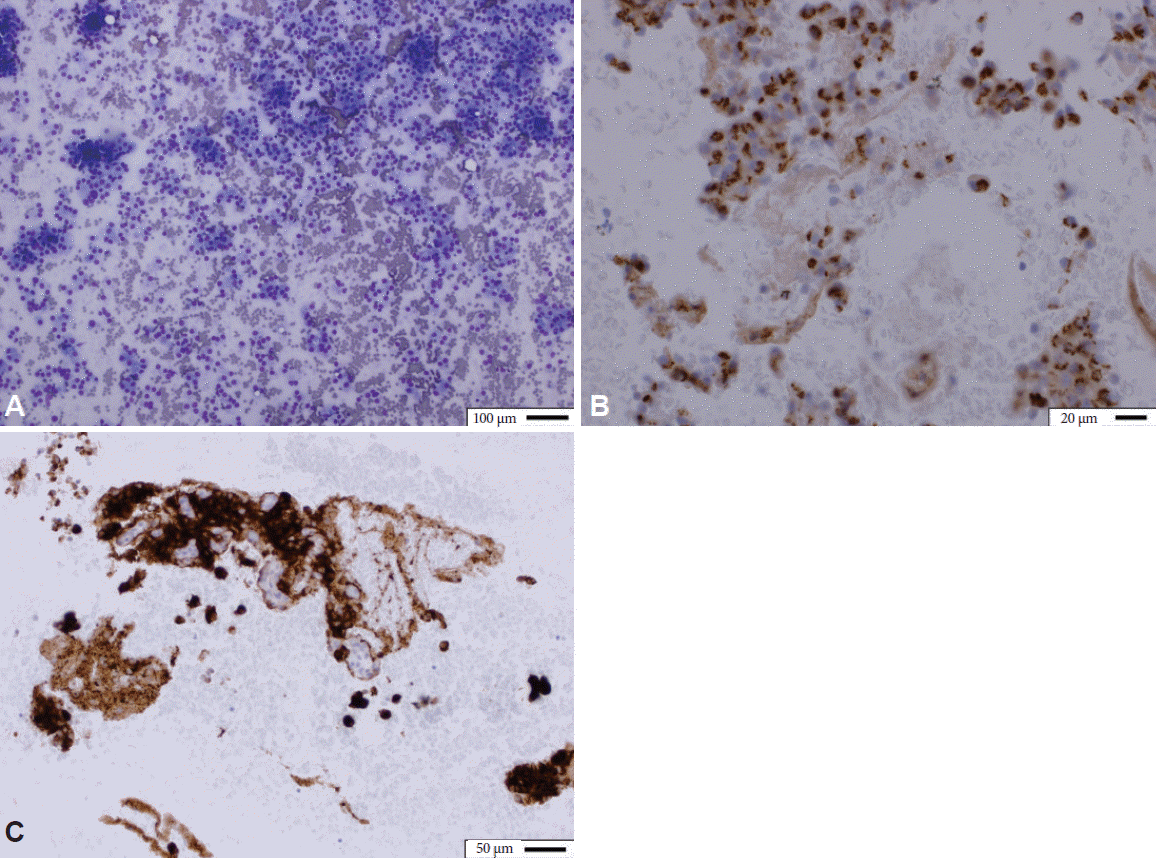
A 66-year-old Caucasian man with decompensated heart failure, asthma, chronic obstructive pulmonary disease, post-traumatic stress disorder, and atrial fibrillation on anti-coagulation was initially referred for management of post-cholecystectomy biliary strictures, which were treated with endoscopic retrograde cholangiopancreatography, and placement of biliary stents. Magnetic resonance imaging performed at that time showed an incidental pancreatic head mass. Subsequently, he underwent (EUS), which demonstrated a 14×12 mm, well defined, hypoechoic lesion within the pancreatic head. EUS-guided fine needle aspiration demonstrated a monomorphic population of neoplastic cells diffusely and strongly positive for CD56, synaptophysin, and chromogranin, compatible with a neuroendocrine tumor (Fig. 1). An octreotide scan showed no evidence of metastatic disease. As the patient was asymptomatic, he was followed conservatively for a non-functioning insulinoma. Six months later, however, the patient was triaged for fasting hypoglycemia (blood sugar levels as low as 45 mg/dL) in tandem with neuroglycopenic symptoms. His C-peptide level was noted to be 7.47 (normal range, 0.80 to 3.85) and his insulin level was 32.8 mIU/L (normal range, 1.9 to 23.0) at the time of hypoglycemia, consistent now with a functioning insulinoma, prompting intervention. Medical therapy with diazoxide (50 mg twice daily) resulted in hypotension, and acute kidney injury necessitating discontinuation. Treatment with octreotide (150 mcg subcutaneously three times daily) did not resolve the hypoglycemic episodes. Although the tumor was resectable, the patient was deemed inappropriate for surgery due to his comorbidities. EUS-guided ethanol ablation was therefore considered. The lesion was injected with 1 mL of ethanol (in 4 divided doses) using three-dimensional targeting, resulting in a hyperechoic blush within the margins of the tumor (Supplementary Video 1 [available online at
http://www.e-ce.org/]). Following the procedure, the patient’s fasting glucose, insulin level, and C-peptide normalized. He had no post-procedural complications or further hypoglycemic attacks throughout 6 weeks of follow-up, confirming complete remission.
Surgical enucleation of tumor, the mainstay of treatment for sporadic insulinoma is associated with a high morbidity (10% to 43%) and a mortality of up to 4%, particularly in the elderly, and those with comorbid conditions [1-3]. EUS-guided ethanol ablation of insulinoma offers a safe, effective, and less invasive alternative to surgery with shorter hospital admissions [2,3]. It has been primarily viewed as an intervention aimed at symptomatic amelioration rather than definitive oncologic therapy [2]. It has also been suggested as an option in recurrent or metastatic insulinoma, where re-operation or radical resection is challenging [2-4]. Post-procedural complications include mild pancreatitis as well as medically controlled ulcer, and hematoma of the duodenal wall [5,6]. This case report adds to only a handful of documented cases treated successfully with EUS-guided ethanol ablation and provides a video demonstrating three dimensional targeting, the latter representing a useful technical approach. Careful patient selection is imperative to optimize outcomes, as small lesions or those in proximity to blood vessels could pose technical difficulties [3]. The possibility of late relapse needing re-intervention, incomplete ablation, and risk of metastasis are other inherent risks [2]. Long-term follow-up is needed to determine the enduring benefits of therapy as well as the potential for malignant transformation [2].
REFERENCES
1. Grover AC, Skarulis M, Alexander HR, et al. A prospective evaluation of laparoscopic exploration with intraoperative ultrasound as a technique for localizing sporadic insulinomas. Surgery. 2005; 138:1003–1008.

2. Levy MJ, Thompson GB, Topazian MD, Callstrom MR, Grant CS, Vella A. US-guided ethanol ablation of insulinomas: a new treatment option. Gastrointest Endosc. 2012; 75:200–206.

3. Qin SY, Lu XP, Jiang HX. EUS-guided ethanol ablation of insulinomas: case series and literature review. Medicine (Baltimore). 2014; 93:e85.
4. Lee MJ, Jung CH, Jang JE, et al. Successful endoscopic ultrasound-guided ethanol ablation of multiple insulinomas accompanied with multiple endocrine neoplasia type 1. Intern Med J. 2013; 43:948–950.

5. Jürgensen C, Schuppan D, Neser F, Ernstberger J, Junghans U, Stölzel U. EUS-guided alcohol ablation of an insulinoma. Gastrointest Endosc. 2006; 63:1059–1062.

6. Deprez PH, Claessens A, Borbath I, Gigot JF, Maiter D. Successful endoscopic ultrasound-guided ethanol ablation of a sporadic insulinoma. Acta Gastroenterol Belg. 2008; 71:333–337.
Supplementary Material
Video 1. EUS-guided ethanol ablation of an insulinoma utilizing three-dimensional targeting (http://dx.doi.org/10.5946/ce.2015.144.v001).
Fig. 1.
(A) Cytologic preparations demonstrate a monomorphous population of neoplastic cells with neuroendocrine features Diff-Quik stain (×20) highlighting typical 3-dimensional clusters. (B) Chromogranin (×100) and (C) synaptophysin immunohistochemical stains both positive for the genetic markers of neuroendocrine differentiation (×40).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download