Abstract
Hemobilia is a rare gastrointestinal bleeding, usually caused by injury to the bile duct. Hemobilia after endoscopic retrograde cholangiopancreatography (ERCP) is generally self-limiting and patients will spontaneously recover, but some severe and fatal hemorrhages have been reported. ERCP-related bowel or bile duct perforation should be managed promptly, according to the type of injury and the status of the patient. We recently experienced a case of late-onset severe hemobilia in which the patient recovered after endoscopic biliary stent insertion. The problem was attributable to ERCP-related bile duct perforation during stone removal, approximately 5 weeks prior to the hemorrhagic episode. The removal of the stent was performed 10 days before the onset of hemobilia. The bleeding was successfully treated by two sessions of transarterial coil embolization.
Hemobilia after therapeutic endoscopy is most often caused by traumatic injury, such as manipulation of the instruments during the procedure. A classic triad of upper abdominal pain, upper gastrointestinal (GI) bleeding, and jaundice is present in patients with hemobilia, but only 22% of patients have all three symptoms. The onset of hemobilia commonly occurs within 4 weeks of bile duct injury, but the range of occurrence is widely variable [1]. Risk factors for delayed onset hemobilia include bile stasis, hepatic necrosis, and the formation of a slowly expanding pseudoaneurysm. Biliary stent-related hemobilia following an endoscopic procedure has been reported much less often than hemobilia attributable to iatrogenic causes related to percutaneous liver procedures. Until now, four cases related to an endoscopic and percutaneous approach have been reported and almost all of them occurred immediately or few days after the procedure. Endoscopic retrograde cholangiopancreatography (ERCP) is a relatively invasive procedure and may cause hemobilia; however, it can be a useful tool for diagnosis and management of hemobilia. ERCP-related biliary or duodenal perforation must be managed promptly, taking into account the patient’s comorbidities and the type of perforation. Endoscopic treatment with a biliary plastic stent can be done in cases of bile duct perforation, in patients of an advanced age. To our knowledge, delayed hemobilia secondary to endoscopic stent inserted for ERCP-related perforation has not been reported until now. We recently managed a case of ERCP-related bile duct perforation after the removal of common bile duct (CBD) stones that caused cholangitis. The perforation was successfully managed by endoscopic insertion of plastic biliary stents, but severe hemobilia was encountered approximately 5 weeks after the stent insertion and a repeated episode at 10 days after the removal of stents. The hemobilia was successfully treated by two sessions of percutaneous transarterial embolization.
A 75-year-old female patient presented to our emergency center with abdominal pain for the last 7 days. Her past medical history included removal of CBD stones and a laparoscopic cholecystectomy for gallbladder stones in SAM Anyang Hospital, 3 years before the current admission and total abdominal hysterectomy for uterine myoma 10 years before this admission. For the last 5 years, she received medication for hypertension and osteoporosis. Her vital signs were: blood pressure 110/70 mm Hg, pulse rate 88 beats per minute, respiratory rate of 20 breaths per minute, and body temperature, 37.9°C. Physical examination showed an acute ill-looking appearance and tenderness without rebound in epigastrium and was otherwise unremarkable. Initial laboratory findings were as follows: hemoglobin (Hb), 13.4 g/dL; aspartate aminotransferase (AST)/alanine aminotransferase (ALT), 522/1,236 IU/L; alkaline phosphatase (ALP), 518 IU/L; total bilirubin, 3.5 mg/dL; blood urea nitrogen (BUN)/creatinine (Cr), 30/1.7 mg/dL; and C-reactive protein, 115.2 mg/L. Abdominal non-enhanced computed tomography (CT) due to renal impairment revealed a 15 mm calcified stone in her CBD, with proximal bile duct dilation and surgical clips around the gallbladder bed from the cholecystectomy (Fig. 1). Intravenous antibiotics were administered and ERCP was performed. After extended biliary sphincterotomy and transpapillary balloon dilation up to 15 mm, several brown, fragmented stones were removed using a basket and retrieval balloon. The total procedure time was approximately 50 minutes and her vital signs including oxygen saturation were stable during the procedure. Severe abdominal distension with pain was observed just after the procedure and a physical examination showed severe tenderness with rebound in the right upper quadrant. A post-ERCP chest radiography obtained 15 minutes after the procedure showed a huge pneumoperitoneum in the right subphrenic area. After the removal of the nasobiliary drainage tube, emergency upper GI endoscopy was immediately performed (30 minutes after the ERCP) in order to detect the perforation site. However, no perforation was observed in the hollow viscus or sphincterotomy sites and it was assumed that the perforation was due to bile duct injury above the pancreas. A nasobiliary drainage tube used to lower the abdominal pressure after an injured bile duct is uncomfortable when used for several days in comparison with a biliary plastic stent. Consequently, it was decided that the patient will benefit from biliary stenting. Two indwelling biliary plastic stents (one 8 cm pigtail type, the other 10 cm straight type) were inserted into the right hepatic duct (Fig. 2). We then informed the patient and her family about the possibility of an emergent surgery if her vital signs and symptoms will deteriorate. Patient was recommended to withhold oral intake and received intravenous fluid with antibiotics for 4 days after the insertion of the stents. Clinical course was favorable; free air nearly disappeared and she resumed a liquid diet 6 days later. She was discharged 9 days after stone removal and the two stents were removed approximately 3 weeks later in a follow-up visit, as an outpatient. She continued to improve and had not experienced abdominal pain for 4 weeks after discharge. She revisited our emergency center at that time, because of massive hematemesis. Her vital signs were: blood pressure 80/50 mm Hg, pulse rate 107 beats per minute, respiratory rate 18 breaths per minute, and body temperature 36.8°C. She was very pale, anicteric, lethargic, and mildly disoriented. On physical examination, tenderness without rebound in the epigastric region was observed, but otherwise the results were unremarkable. Laboratory findings were as follows: Hb, 7.6 g/dL; white blood cell count, 10,700/µL; AST/ALT, 59/23 IU/L; and BUN/Cr, 16/0.9 mg/dL. Chest radiography showed no free air and no infiltration in either lung. Urgent upper GI endoscopy showed active bleeding from the ampullary orifice consistent with hemobilia (Fig. 3). Subsequent emergent hepatic angiography was performed while a blood transfusion was administered, in an effort to locate and embolize the source of the bleeding. Selective right hepatic arteriography showed a fistula between the right posterior hepatic artery and the bile duct and the fistula was occluded by coil embolization. Her vital signs stabilized after the procedure and her Hb level increased to 10.2 g/dL, but massive and continuous hematochezia was observed 6 hours after the embolization. Her Hb then dropped to 4.6 g/dL and a second emergent hepatic angiography was performed. An angiogram of the right posterior hepatic artery showed the continued leakage of contrast solution into the bile duct, so further embolization was performed with a total of 10 coils, including the coils used in the first intervention (Fig. 4). She stabilized after another blood transfusion and no further active bleeding was observed for 5 days after the second embolization, at which point she was discharged. She did well during the next 12 months follow-up period.
Iatrogenic ERCP-related hemobilia is most common immediately after or within several days of the procedure; delayed onset hemobilia can be due to continuous, chronic bile duct erosion and inflammation contributing to weakening of the bile duct, with injury to adjacent vessels, and eventual bleeding into the bile duct. Bile duct perforation after ERCP can be a cause of delayed onset hemobilia if the lesion is incompletely healed—inflammatory bile duct weakening contributes to vessel injury and leads to bleeding into the bile duct, as in our case. Perforation of the bile duct after ERCP can be conservatively managed in patients with minor bile duct injury, but in the case of a relatively large pneumoperitoneum after ERCP, as in our patient, it can be difficult to decide between surgery and endoscopic therapy with conservative management. After we confirmed through emergent upper GI endoscopic examination that there was no perforation of the bowel or sphincterotomy, biliary plastic stent insertion successfully treated the bile duct perforation. Patient’s pain was relieved and a decrease of free air was evident on chest radiography. The appropriate indwelling time of biliary stents after bile duct perforation is variable, but the appropriate time to remove the stent is more than 6 to 8 weeks. Delayed hemobilia in our patient was probably caused by the ongoing inflammation from incomplete healing of the bile duct. This could contribute to the fistula formation between the bile duct and the hepatic artery and eventually bleeding, even though the patient seemed to have recovered completely at discharge and had no bleeding until 10 days after the removal of the stents. To our knowledge, there is insufficient data for a consensus on the optimal duration of biliary stenting, especially for plastic stents, if ERCP-related bile duct perforation occurs. For metal stents, some researchers advocate a relatively long duration, ranging from 2 months to more than 6 months [2]. Although the removal of the stent was postponed for 6 to 8 weeks, whether hemobilia could have been prevented in this patient is not clear, but a long indwelling time of the stent might be beneficial for a complete healing of the bile duct. Alternatively, direct injury due to the inserted biliary stent is less likely, especially since no bleeding was observed during hospitalization after the insertion of the stents and for 10 days after the removal of the stents. However, it is difficult to appreciate whether the previously inserted two stents (8 and 10 cm in length) were located in the right anterior or posterior hepatic ducts because of the inaccuracy of two-dimensional simple radiographies. There is a possibility that the delayed bleeding was caused by the plastic stents being inserted into the posterior hepatic duct.
Biliary metal stent insertion, mainly performed in cases of malignant biliary obstruction, has been known to cause acute or delayed hemobilia [3,4]. Other cases with pseudoaneurysms attributable to continuous erosion caused by biliary metal stents accompanying symptomatic hypertensive biliopathy have been reported [5-7]. In contrast to metal stents, plastic stents very rarely cause severe hemobilia. Notably though, there was one case of hemobilia which occurred during extraction of a plastic stent by endoscopy, in a patient with metastatic bile duct obstruction caused by ovarian cancer. In another case reported in the literature, hemobilia occurred 2 days after the insertion of a plastic stent via a percutaneous route, in a patient with a Klatskin tumor [8,9]. A third case occurred 3 weeks after stent insertion, because a long (15 cm) biliary plastic stent was used to relieve obstructive jaundice caused by extrinsic compression by large pancreatic pseudocysts that continuously eroded a hepatic artery which resulted in bleeding [10]. In our patient, so the long stent was not used; a 10 cm straight and aother 8 cm pigtail stent were used. To our knowledge, there has been no case report of delayed severe hemobilia due to ERCP-related bile duct perforation, after successful endoscopic management with biliary plastic stent insertion.
Transarterial embolization is the first-line treatment and the success rate is 80% to 100%, according to the most recent literature. If that procedure fails, the next step is surgery, which is rarely necessary and depends on the etiology of hemobilia. Surgery involves direct exploration of the liver with hepatic resection, ligation, and/or ballooning of the bleeding vessel, aneurysm excision, cholecystectomy, and release of the bile duct obstruction [11]. ERCP has also recently played an important role in non-iatrogenic hemobilia because hepatobiliary tumors are a predominant cause of non-iatrogenic hemobilia [12].
In summary, we report the first case of severe delayed hemobilia due to ERCP-related bile duct perforation, after successful endoscopic management by stent insertion, in which bleeding occurred 10 days after stent removal and approximately 3 weeks after stent insertion. We believe that if endoscopic stent insertion is performed in cases of ERCP-related bile duct perforation, stent removal may be postponed for more than 6 to 8 weeks, in order to allow for complete healing of the bile duct perforation.
REFERENCES
1. Goffette PP, Laterre PF. Traumatic injuries: imaging and intervention in post-traumatic complications (delayed intervention). Eur Radiol. 2002; 12:994–1021.

2. Lee SM, Cho KB. Value of temporary stents for the management of perivaterian perforation during endoscopic retrograde cholangiopancreatography. World J Clin Cases. 2014; 2:689–697.

3. Nezu Y, Nakaji S, Fujii H, Ishii E, Hirata N. Pseudoaneurysm caused by a self-expandable metal stent: a report of three cases. Endoscopy. 2014; 46:248–251.

4. Jeong HS, Shin TB, Hwang JC, Bae JI, Kim CW. Interventional management of delayed and massive hemobilia due to arterial erosion by metallic biliary stent. J Korean Soc Radiol. 2012; 66:27–33.

5. Rai R, Rose J, Manas D. Potentially fatal haemobilia due to inappropriate use of an expanding biliary stent. World J Gastroenterol. 2003; 9:2377–2378.

6. Watanabe M, Shiozawa K, Mimura T, et al. Hepatic artery pseudoaneurysm after endoscopic biliary stenting for bile duct cancer. World J Radiol. 2012; 4:115–120.

7. Layec S, D’Halluin PN, Pagenault M, Bretagne JF. Massive hemobilia during extraction of a covered self-expandable metal stent in a patient with portal hypertensive biliopathy. Gastrointest Endosc. 2009; 70:555–556.

8. Conio M, Caroli-Bosc FX, Buckley M, Chiaramondia M, D’Addazio G, Munizzi F. Massive hematobilia after extraction of plastic biliary endoprosthesis. J Clin Gastroenterol. 1997; 25:706.

9. Park JY, Ryu H, Bang S, Song SY, Chung JB. Hepatic artery pseudoaneurysm associated with plastic biliary stent. Yonsei Med J. 2007; 48:546–548.

10. Wolters F, Ryan B, Beets-Tan R, Dejong C. Delayed massive hemobilia after biliary stenting. Endoscopy. 2003; 35:976–977.

11. Bloechle C, Izbicki JR, Rashed MY, et al. Hemobilia: presentation, diagnosis, and management. Am J Gastroenterol. 1994; 89:1537–1540.
Fig. 1.
(A, B) Abdominal non-enhanced computed tomography shows 15 mm-sized calcified stone in common bile duct (black arrow) and surgical clips are seen at the level of common haptic duct (white arrow).
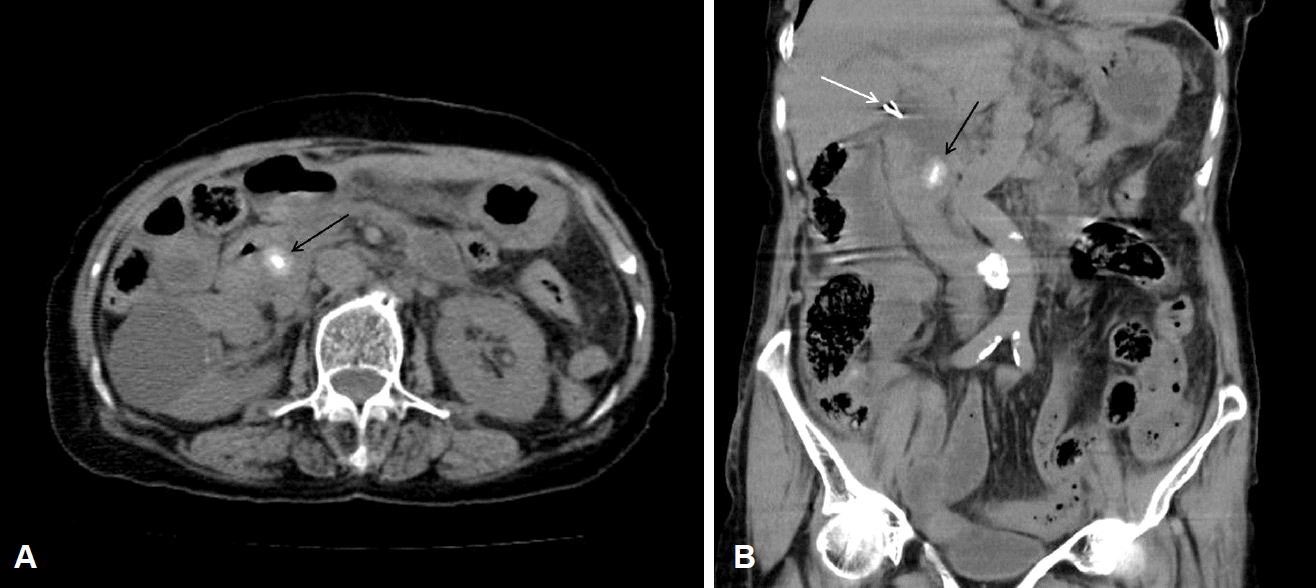
Fig. 2.
Post-endoscopic retrograde cholangiopancreatography. (A) Chest radiography shows a large pneumoperitoneum under the right hemidiaphragm and nasobiliary drainage tube is also seen in the common bile duct is (black arrow) with air-biliarygram in the right anterior hepatic duct. (B) Two plastic biliary stents (one pigtail type [white arrow], the other straight type) are deeply located into the right hepatic duct.
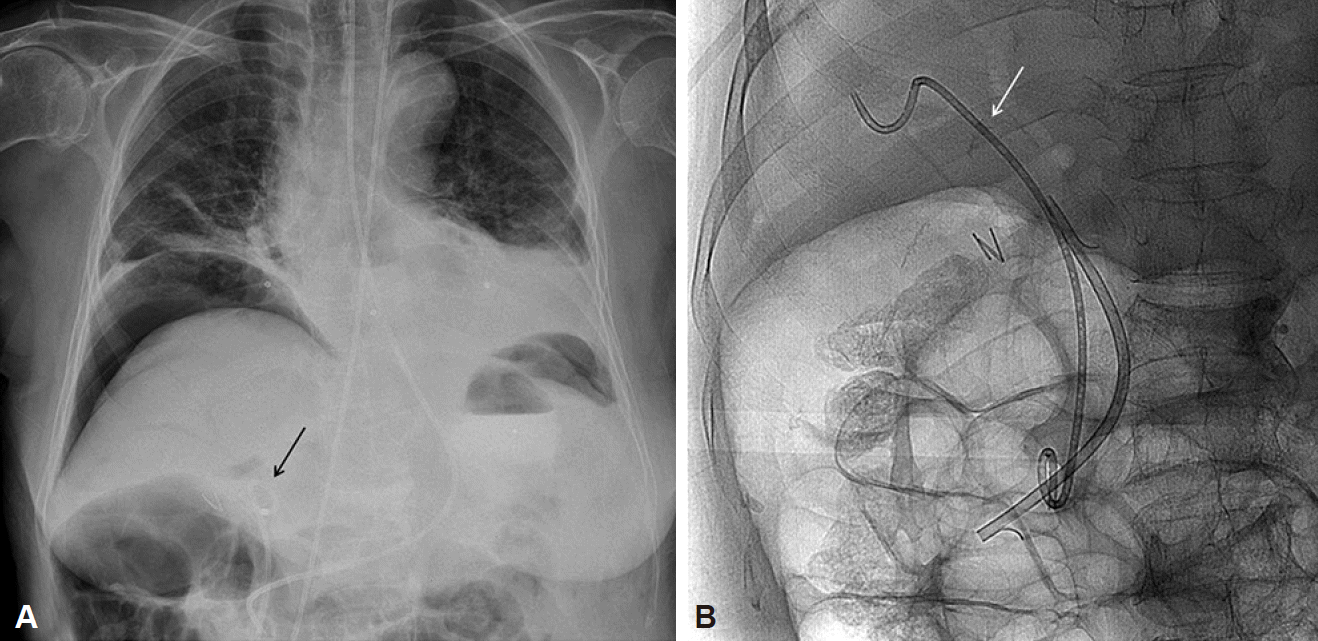
Fig. 3.
(A, B) Emergent upper gastrointestinal endoscopy shows profuse active bleeding in the duodenum and the identified source of bleeding was the papillary orifice (white arrow), which is consistent with hemobilia.
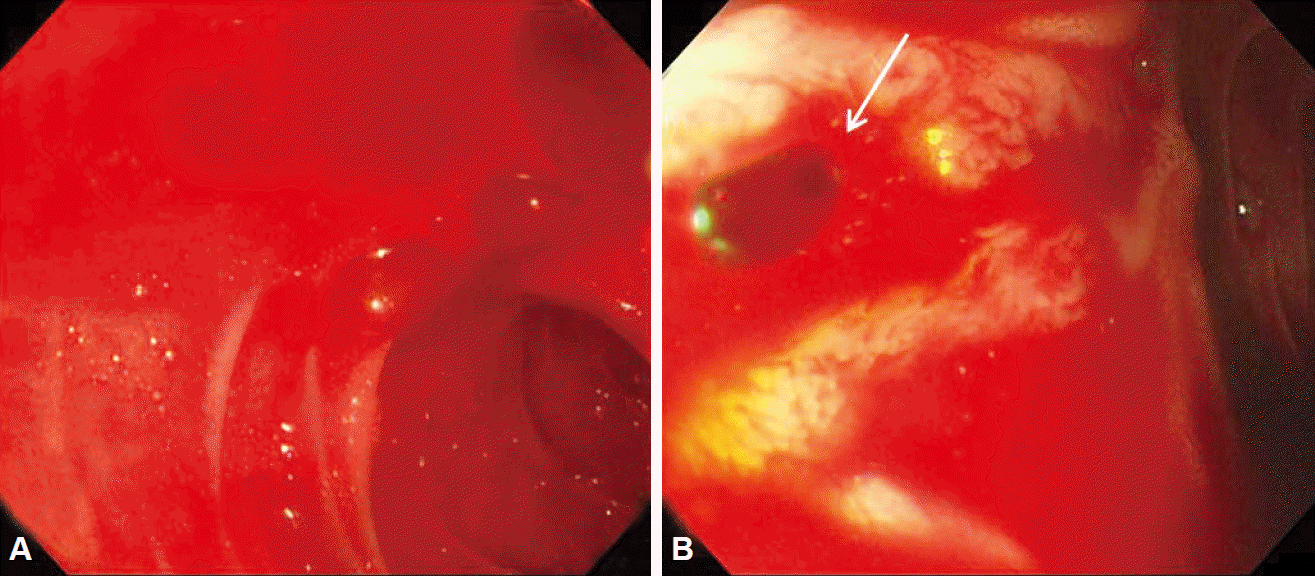
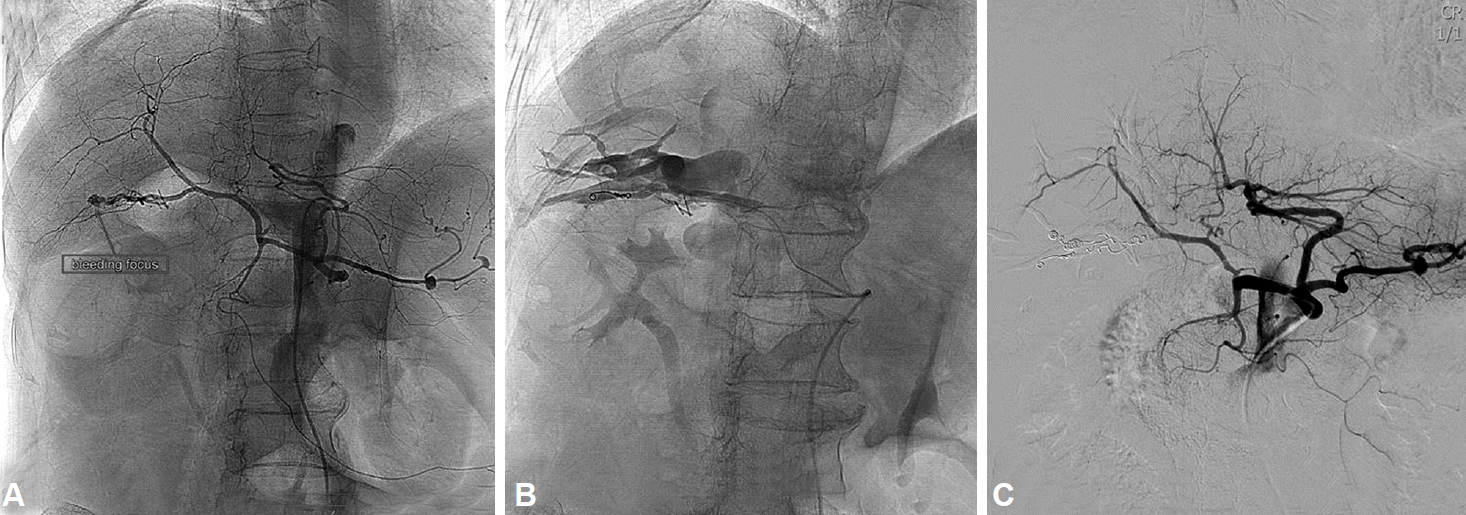
Fig. 4.
Transarterial angiogram via celiac artery. (A) The left panel shows the extravasation of contrast material in the right posterior hepatic artery (RPHA), which is suggestive for active bleeding. (B) Cholangiogram of the right posterior hepatic duct is obtained through the arteriobiliary fistula near the RPHA. (C) After two sessions of embolization by using multiple coils, no further contrast extravasation is seen in right panel.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download